Generic Name
Tc-99M
Brand Names
Ultra-Technekow V4, TechneLite
FDA approval date: November 01, 1975
Classification: Radioactive Diagnostic Agent
Form: Injection
What is Ultra-Technekow V4 (Tc-99M)?
The Technelite generator is a source of sodium pertechnetate Tc 99m for use in the preparation of FDA-approved diagnostic radiopharmaceuticals, as described in the labeling of these diagnostic radiopharmaceutical kits. Sodium Pertechnetate Tc 99m Injection is used IN ADULTS as an agent for: Thyroid Imaging Salivary Gland Imaging Urinary Bladder Imaging for the detection of vesico-ureteral reflux. Nasolacrimal Drainage System Imaging Sodium Pertechnetate Tc 99m Injection is used IN CHILDREN as an agent for: Thyroid Imaging Urinary Bladder Imaging for the detection of vesico-ureteral reflux.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Ultra-Technekow (Technetium Tc-99m)
1DESCRIPTION
The Ultra-Technekow
This terminally sterilized generator provides a closed system for the production of sterile metastable technetium Tc-99m, which is produced by the decay of molybdenum Mo-99. Incorporated between the column outlet and the collection vial is a sterile 0.22 micrometer filter. Sterile, non-pyrogenic isotonic solutions of Sodium Pertechnetate Tc 99m Injection in 0.9% Sodium Chloride Injection can be obtained conveniently by periodic aseptic elution of the generator. These solutions should be clear, colorless, and free from any particulate matter. The Sodium Pertechnetate Tc 99m Injection is suitable for intravenous injection and direct instillation.
The carrier-free solution may be used as is, or diluted to the proper concentration. Over the life of the generator, an elution will contain an amount of technetium Tc-99m in direct proportion to the quantity of Mo-99 decay since the previous elution of the generator. The quantity of Tc-99m in the eluate is determined by quantity of Mo-99 on the column, and the elapsed time between elutions.
Each eluate of the generator should not contain more than the USP limit of 0.15 kilobecquerel molybdenum Mo-99 per megabecquerel technetium Tc-99m (0.15 microcurie Mo-99 per millicurie Tc-99m) per administered dose at the time of administration and an aluminum ion concentration of not more than 10 micrograms per milliliter of the generator eluate, both of which must be determined by the user before administration.
Since the eluate does not contain an antimicrobial agent, it should not be used after 12 hours from the time of generator elution.
1.1Physical Characteristics
Technetium Tc-99m decays by isomeric transition with a physical half-life of 6 hours. The principal photon that is useful for detection and imaging studies is listed in Table 1.
Table 1. Principal Radiation Emission Data
1.2External Radiation
The specific gamma ray constant for technetium Tc-99m is 0.795 R/hr-mCi at 1 cm. The first half-value layer is 0.023 cm of lead (Pb). A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of 0.27 cm thickness of Pb will attenuate the radiation emitted by a factor of about 1000.
Table 2. Radiation Attenuation by Lead Shielding
Molybdenum Mo-99 decays to technetium Tc-99m with a molybdenum Mo-99 half-life of 2.75 days, or 66 hours (see Table 3). The physical decay characteristics of molybdenum Mo-99 are such that only 88.6% of the decaying molybdenum Mo-99 atoms form technetium Tc-99m. Generator elutions may be made at any time, but the amount of technetium Tc-99m available will depend on the interval measured from the last elution. Approximately 47% of the maximum available technetium Tc-99m is reached after 6 hours and 95% after 23 hours. To correct for physical decay of molybdenum Mo-99 and technetium Tc-99m, the fractions that remain at selected intervals of time are shown in Tables 3 and 4.
Table 3. Physical Decay Chart; Molybdenum Mo-99, Half-Life 66 Hours
Table 4. Physical Decay Chart; Technetium Tc-99m, Half-Life 6 Hours
*Calibration Time
2CLINICAL PHARMACOLOGY
The pertechnetate ion distributes in the body similarly to the iodide ion but is not organified when trapped in the thyroid gland. Pertechnetate concentrates in the thyroid gland, salivary glands, stomach and choroid plexus. After intravenous administration it gradually equilibrates with the extracellular space. A fraction is promptly excreted via the kidneys.
Following the administration of Sodium Pertechnetate Tc 99m as an eye drop, the drug mixes with tears within the conjunctival space. Within seconds to minutes it leaves the conjunctival space and escapes into the inferior meatus of the nose through the nasolacrimal drainage system. During this process the pertechnetate ion passes through the canaliculi, the lacrimal sac and the nasolacrimal duct. In the event of any anatomical or functional blockage of the drainage system there will be a backflow resulting in tearing (epiphora). Thus the pertechnetate escapes the conjunctival space in the tears.
While the major part of the pertechnetate escapes within a few minutes of normal drainage and tearing, it has been documented that there is some degree of transconjunctival absorption with turnover of 1.5% per minute in normal individuals, 2.1% per minute in patients without any sac and 2.7% per minute in patients with inflamed conjunctiva due to chronic dacryocystitis. Individual values may vary but these rates are probably representative and indicate that the maximum possible pertechnetate absorbed will remain below one thousandth of that used in other routine diagnostic procedures.
3INDICATIONS AND USAGE
The Ultra-Technekow™ V4 generator is a source of sodium pertechnetate Tc 99m for use in the preparation of FDA-approved diagnostic radiopharmaceuticals, as described in the labeling of these diagnostic radiopharmaceutical kits.
Sodium Pertechnetate Tc 99m is used
Thyroid Imaging
Sodium Pertechnetate Tc 99m is used
Thyroid Imaging
4CONTRAINDICATIONS
None.
5WARNINGS
Radiation risks associated with the use of Sodium Pertechnetate Tc 99m are greater in pediatric patients than in adults and, in general, the younger the patient the greater the risk owing to greater absorbed radiation doses and longer life expectancy. These greater risks should be taken firmly into account in all benefit risk assessments involving pediatric patients.
Long-term cumulative radiation exposure may be associated with an increased risk of cancer.
Only use generator eluant specified for use with the Ultra-Technekow
6PRECAUTIONS
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
As in the use of any radioactive material, care should be taken to minimize radiation exposure to the patient consistent with proper patient management and to ensure minimum radiation exposure to occupational workers.
After the termination of the nasolacrimal imaging procedure, blowing the nose and washing the eyes with sterile distilled water or an isotonic sodium chloride solution will further minimize the radiation dose.
Since the eluate does not contain an antimicrobial agent, it should not be used after 12 hours from time of generator elution.
6.1Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic or mutagenic potential or whether Sodium Pertechnetate Tc 99m may affect fertility in males or females.
6.2Pregnancy
In animal reproductive studies, Sodium Pertechnetate Tc 99m (as free pertechnetate) has been shown to cross the placental barrier. It is not known whether Sodium Pertechnetate Tc 99m can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity.
Sodium Pertechnetate Tc 99m should be given to pregnant women only if the expected benefits to be gained clearly outweigh the potential hazards.
Ideally, examinations using radiopharmaceutical drug products - especially those elective in nature - of women of childbearing capability should be performed during the first ten days following the onset of menses.
6.3Nursing Mothers
Technetium Tc-99m is excreted in human milk during lactation, therefore, formula-feedings should be substituted for breast-feedings.
6.4Pediatric Use
See
7ADVERSE REACTIONS
Allergic reactions including anaphylaxis have been reported infrequently following the administration of Sodium Pertechnetate Tc 99m.
8DOSAGE AND ADMINISTRATION
Sodium Pertechnetate Tc 99m is administered by intravenous injection. When imaging the nasolacrimal drainage system, instill the Sodium Pertechnetate Tc 99m by the use of a device such as a micropipette or similar method which will ensure the accuracy of the dose.
For imaging the urinary bladder and ureters (direct isotopic cystography), the Sodium Pertechnetate Tc 99m is administered by direct instillation aseptically into the bladder via a urethral catheter, following which the catheter is flushed with approximately 200 mL of sterile saline directly into the bladder.
The suggested dose ranges employed for various diagnostic indications in the average ADULT PATIENT (70 kg) are as follows:
Vesico-ureteral imaging: 18.5 to 37 MBq (0.5 to 1 mCi)
The recommended dosages in PEDIATRIC PATIENTS are:
Vesico-ureteral imaging: 18.5 to 37 MBq (0.5 to 1 mCi)
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. If the solution is discolored, discontinue use of the generator immediately. The solution to be administered as the patient dose should be clear, colorless, and contain no particulate matter.
8.1Radiation Dosimetry
The estimated absorbed radiation doses to an average
Table 5. Absorbed Radiation Doses from Intravenous Injection
To obtain radiation absorbed dose in rads (30 mCi dose) from the above table, divide individual organ values by a factor of 10 (does not apply for effective dose).
Table 6. Pediatric Absorbed Radiation Doses (mGy) from Intravenous Injection
To obtain radiation absorbed dose in rads (30 mCi dose) from the above table, divide individual organ values by a factor of 10 (does not apply for effective dose).
The estimated absorbed radiation doses to an ADULT patient from the nasolacrimal imaging procedure using a maximum dose of 3.7 megabecquerels (100 microcuries) of Sodium Pertechnetate Tc 99m are shown in Table 7.
Table 7. Absorbed Radiation Doses from Dacryoscintigraphy
*Assuming no blockage of draining system.
In pediatric patients, an average 30 minute exposure to 37 MBq (1 mCi) of Tc-99m pertechnetate following instillation for direct cystography, will result in the following estimated radiation doses:
Table 8. Absorbed Radiation Doses from Cystography (PEDIATRIC)
9HOW SUPPLIED
The Ultra-Technekow
Catalog No.
9010 37 gigabecquerels (1.0 curie)
9015 55.5 gigabecquerels (1.5 curies)
9020 74 gigabecquerels (2.0 curies)
9025 92.5 gigabecquerels (2.5 curies)
9030 111 gigabecquerels (3.0 curies)
9035 129.5 gigabecquerels (3.5 curies)
9051 185 gigabecquerels (5.0 curies)
9060 222 gigabecquerels (6.0 curies)
9075 277.5 gigabecquerels (7.5 curies)
9110 407 gigabecquerels (11.0 curies)
9140 518 gigabecquerels (14.0 curies)
9160 592 gigabecquerels (16.0 curies)
9190 703 gigabecquerels (19.0 curies)
Each generator is supplied with the following components for the elution of the generator:
1 - Technestat
1 - Package Insert
SUPPLIED SEPARATELY
30 - Evacuated Collecting Vials, 30 mL, sterile, non-pyrogenic, supplied with:
90 - Radioactive Materials Labels – Collection Vial (30 en, 30 fr, 30 es)
90 - Radioactive Materials Labels – Elution Shield (30 en, 30 fr, 30 es)
1 - Package Insert
30 - Generator Eluant, 0.9% Sodium Chloride Injection, sterile, non-pyrogenic, available in 5 mL, 10 mL, or 20 mL volumes, with 1 package insert. The eluant does not contain an antimicrobial agent. Each milliliter of Generator Eluant contains 9 milligrams of Sodium Chloride.
Storage
Store generator and Sodium Pertechnetate Tc 99m solution at controlled room temperature 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Expiration Date
The generator should not be used after the expiration date stated on the label.
The expiration time of the Sodium Pertechnetate Tc 99m solution is not later than 12 hours after time of elution. If the eluate is used to reconstitute a kit, the radiolabeled kit should not be used after 12 hours from the time of generator elution or after the expiration time stated on the labeling for the prepared drug, whichever is earlier.
9.1Directions for Use of the Technetium Tc 99m Generator
NOTE 1: Immediately upon delivery, the generator should be placed within a minimum of one-inch of lead shielding in such a manner
NOTE 2: Wear waterproof gloves during the elution procedure and during subsequent reconstitution of kits with the eluate.
NOTE 3: Use a shielded syringe to withdraw patient dose or to transfer Sodium Pertechnetate Tc 99m into mixing vials during kit
NOTE 4: The needles in the generator are sterile beneath their covers, and the generator has been cleaned underneath the top cover.
Eluting the generator every 24 hours will provide optimal amounts of Sodium Pertechnetate Tc 99m. However, the generator may be eluted whenever sufficient amounts of technetium Tc-99m have accumulated within the column.
For Example
Elution
- Lift the generator by its handle and place it inside the auxiliary shield. Move the handle so that it is not covering the generator top by pushing it off to the side in between the generator and the auxiliary shield.
- Remove and store the elution hood cover. Place the auxiliary shield top onto the top of the generator and align it with the elution hood.
- Remove and store the tip cap plugs from the needles.
- Remove the flip-top cap of the eluant vial; disinfect the stopper with a bacteriocide such as 70% isopropyl alcohol, allowing the stopper to dry before use. Invert the eluant vial and place stopper first into the saline vial alignment insert. Place the saline vial alignment insert and vial into the saline port of the auxiliary shield top and firmly push down the eluant vial until the stopper is punctured and seated at the base of the eluant needles.
- Place the saline shield on top of the auxiliary shield top to cover the eluant vial.
- Remove the flip-top cap of an evacuated vial; disinfect the stopper, allowing the stopper to dry before use. Place the evacuated vial into the elution tool.
- Position the shielded evacuated vial by carefully lowering the elution tool into place on the elution needle. Piercing the septum of the evacuated vial with the elution needle will begin the elution process.
- Wait until the evacuated vial has completely filled itself. This may take a few minutes.
- NOTE: Do not use generator eluate if its appearance is discolored, and discontinue use of the generator.
- Remove the flip-top cap of the Technestat vial; disinfect the stopper, allowing the stopper to dry before use. Secure the Technestat vial into the Technestat vial holder.
- Carefully remove the elution tool and replace with the shielded Technestat vial.
- Determine the technetium Tc-99m concentration and molybdenum Mo-99 content for dispensing purposes. The generator eluate may be assayed using an appropriate detection system. The manufacturer’s instructions for operation of the instrument/equipment should be followed for measurement of Technetium Tc-99m and Molybdenum Mo-99 activity.
- Determine the aluminum ion concentration of the eluate.
Subsequent Elutions
- Remove the saline shield and then remove saline vial alignment insert from the saline port to remove the eluant vial from the eluant needles. Remove the vial from the saline vial alignment insert and reuse the saline vial alignment insert for subsequent elutions.
- Remove the shielded Technestat vial by carefully lifting the Technestat vial shield from the elution needle.
- Repeat steps 4 through 12 of the Elution procedure above.
Vacuum Loss
If the vacuum in the collecting vial is lost, do not attempt to re-evacuate the vial, but discard and use a new collecting vial.
Expired Generator Disposal
- Following the life of the generator, remove and dispose of the used Technestat vial and the eluant vial.
- Cover the elution and eluant needles with the stored tip cap plugs.
- Place the stored elution hood cover onto the top of the generator.
- The intact generator assembly should be either returned to Curium US LLC or disposed of in accordance with applicable regulations.
This generator may be received, used and administered only by authorized persons in designated clinical settings. Its receipt, storage, use, transfer and disposal are subject to the regulations and/or appropriate licenses of local competent official organizations.
©2022 Curium US LLC. Ultra-Technekow
Manufactured by:
A901IS
Revised 5/2022
STERILE
CURIUM™
10Principal Display Panel - 37 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
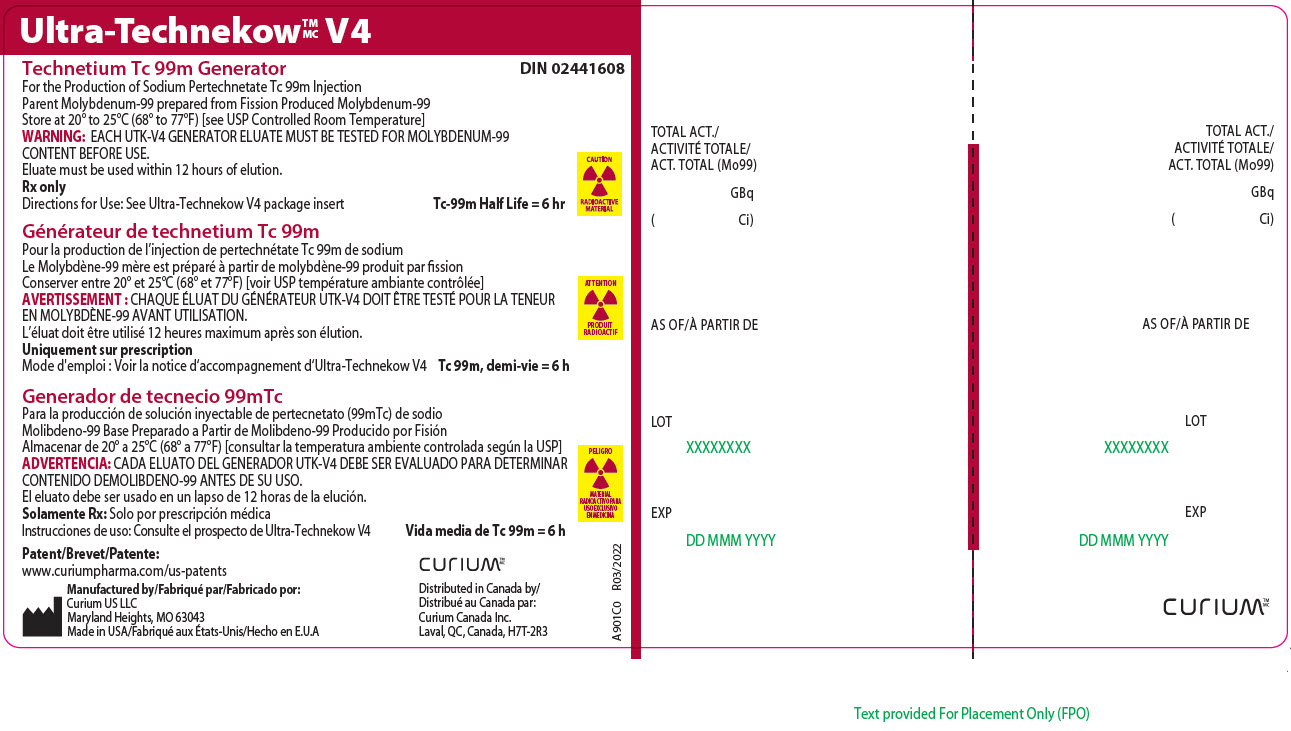
11Principal Display Panel - 55.5 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
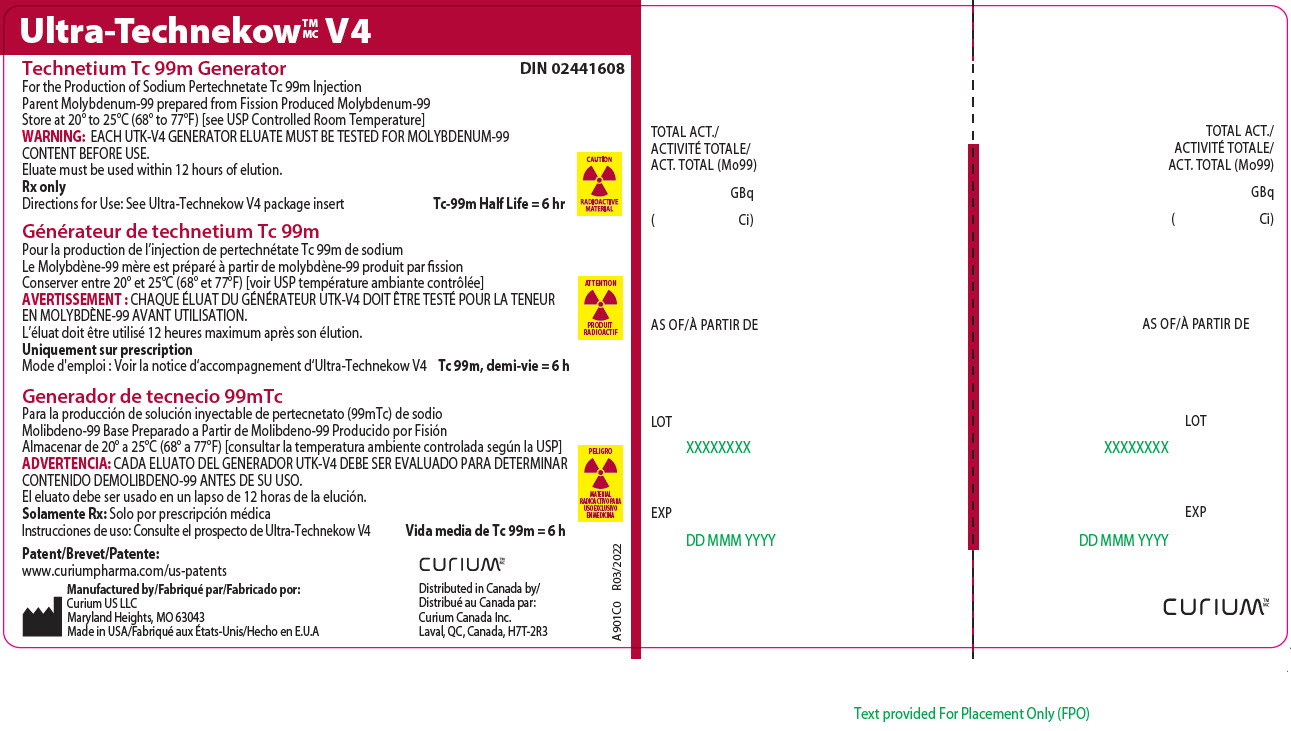
12Principal Display Panel - 74 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
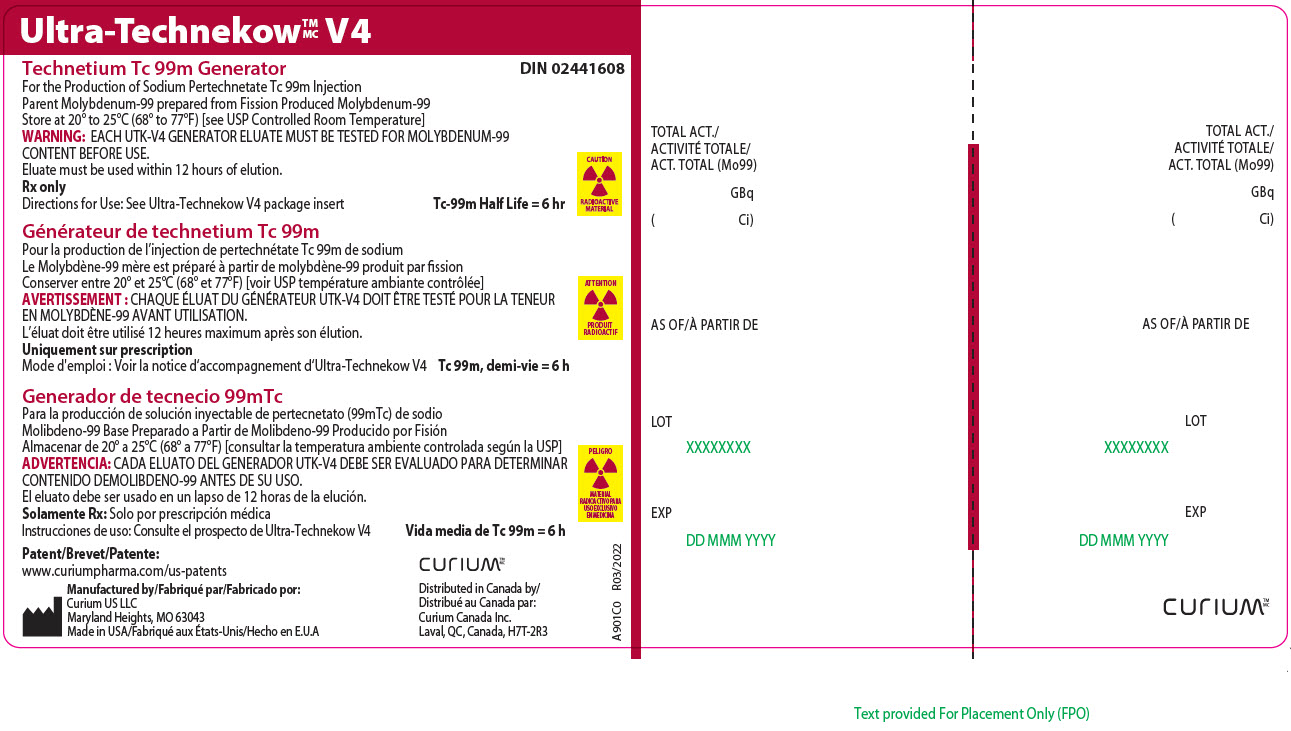
13Principal Display Panel - 92.5 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
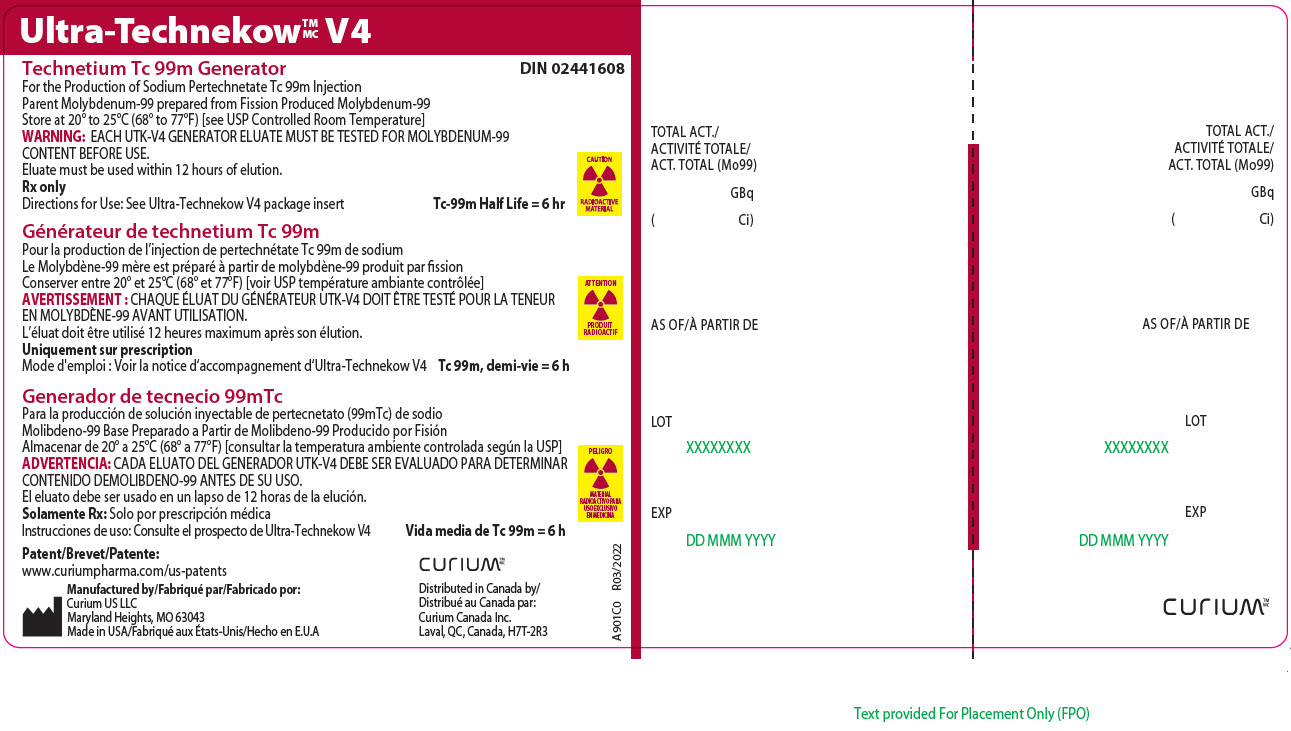
14Principal Display Panel - 111 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
15Principal Display Panel - 129.5 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
16Principal Display Panel - 185 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
17Principal Display Panel - 222 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
18Principal Display Panel - 277.5 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
19Principal Display Panel - 407 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
20Principal Display Panel - 518 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
21Principal Display Panel - 592 gigabecquerels
Ultra-Technekow
For oduction of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP
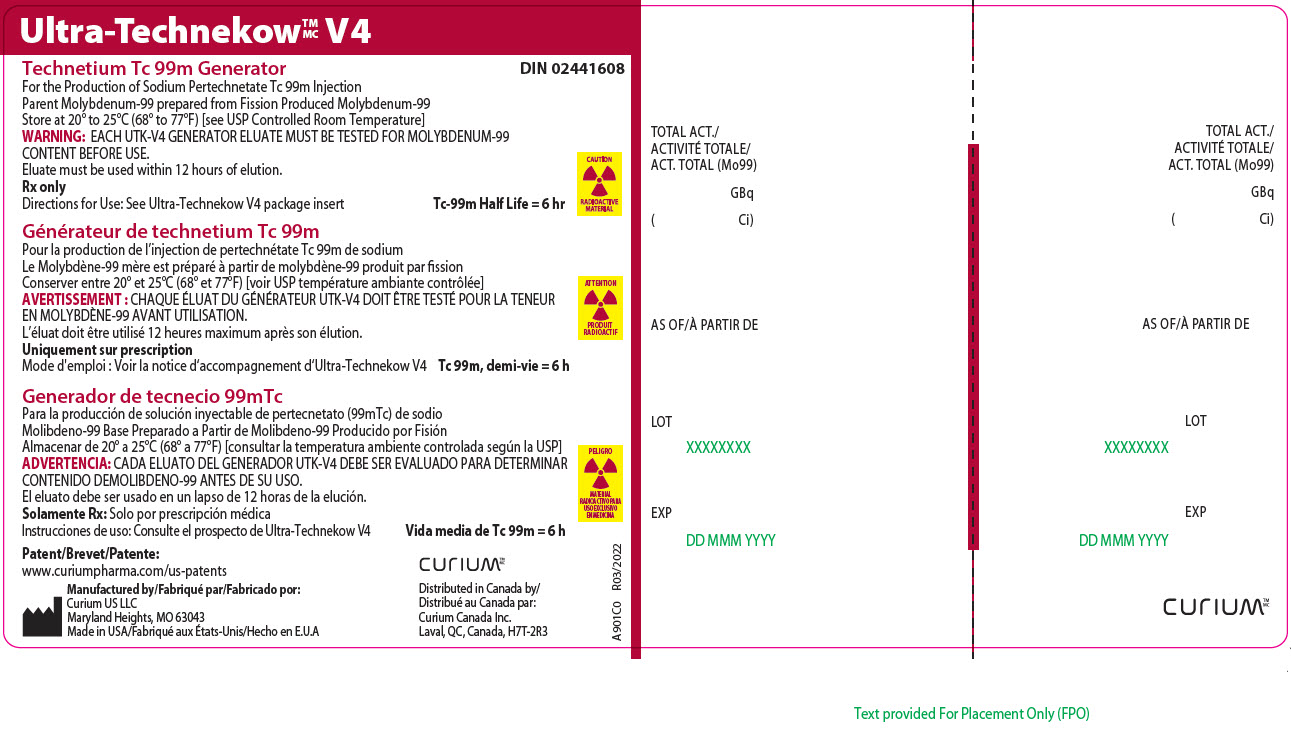
22Principal Display Panel - 703 gigabecquerels
Ultra-Technekow
For the Production of Sodium Pertechnetate Tc 99m Injection
CAUTION
RADIOACTIVE MATERIAL
RADIOACTIVE MATERIAL
Patent/Brevet/Patente:www.curiumpharma.com/us-patents
Manufactured by/Fabriqué par/Fabricado por:Curium US LLC
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
Maryland Heights, MO 63043
Made in USA/Fabriqué aux États-Unis/Hecho en E.U.A
CURIUM
Distributed in Canada by/
A901C0
TOTAL ACT./
AS OF/À PARTIR DE
LOT
EXP