Torisel
What is Torisel (Temsirolimus)?
Approved To Treat
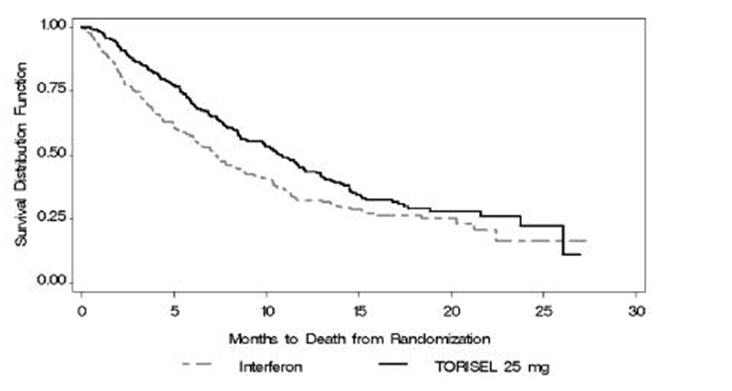
Related Clinical Trials
Summary: Cancer drugs which target the effects of abnormal gene changes are called 'targeted therapies'. This study, called PM.1 or CAPTUR, will include some targeted therapies that are currently available. The purpose of this study is to find out what are the effects on a patient and their cancer when they are given a targeted therapy drug that is specific to an abnormal gene change in their cancer.
Summary: The purpose of the study is to learn from the real world practice of prescribing targeted therapies to patients with advanced cancer whose tumor harbors a genomic variant known to be a drug target or to predict sensitivity to a drug. NOTE: Due to character limits, the arms section does NOT include all TAPUR Study relevant biomarkers. For additional information, contact TAPUR@asco.org, or if a pati...
Summary: This early phase I trial studies the side effects of implanting and removing a microdevice in patients with sarcomas that have spread to other places in the body (metastatic) or have come back (recurrent). Microdevices are rice-sized devices that are implanted into tumor tissue and are loaded with 10 different drugs that are delivered at very small doses, or microdoses, which may only affect a ver...
Related Latest Advances
Brand Information
- Hypersensitivity/Infusion Reactions
- Hepatic Impairment
- Hyperglycemia/Glucose Intolerance
- Infections
- Interstitial Lung Disease
- Hyperlipidemia
- Bowel Perforation
- Renal Failure
- Wound Healing Complications
- Intracerebral Hemorrhage
- OSHA Hazardous Drugs.
(temsirolimus) injection
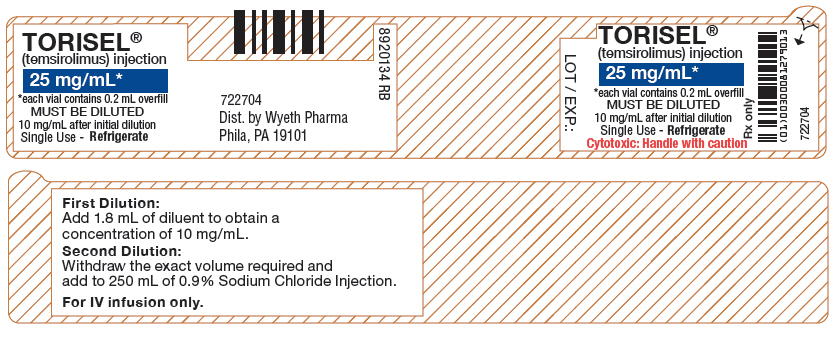
DILUENT
for TORISEL®
Only for dilution of TORISEL
(temsirolimus) injection vial
Single Use - Refrigerate
(temsirolimus) injection
before administration
For intravenous infusion only
Rx only