Lymphoseek
What is Lymphoseek (Tilmanocept)?
Approved To Treat
Related Clinical Trials
Summary: The purpose of this study is to see if Tc 99m Tilmanocept SPECT/CT imaging can be used to identify cardiac sarcoidosis.
Summary: This phase II trial studies how well personalized neck radiation therapy directed by sentinel lymph node biopsy (SLNB) works in treating patients with oral cavity squamous cell carcinoma (OCSCC). SLNB can be performed as part of standard care for OCSCC. During SLNB, a radiotracer is injected around the tumor. The lymph nodes are then biopsied and tested to see if the tracer injected into the tumor...
Summary: A multi-centre validation study to evaluate whether a new imaging and surgical protocol would work as well as the current gold standard in identifying sentinel nodes in patients with oropharyngeal cancer.
Related Latest Advances
Brand Information
- Lymphatic mapping using a handheld gamma counter to locate lymph nodes draining a primary tumor site in adult and pediatric patients age one month and older with solid tumors for which this procedure is a component of intraoperative management.
- Guiding sentinel lymph node biopsy using a handheld gamma counter in patients with clinically node negative squamous cell carcinoma of the oral cavity, breast cancer or melanoma.
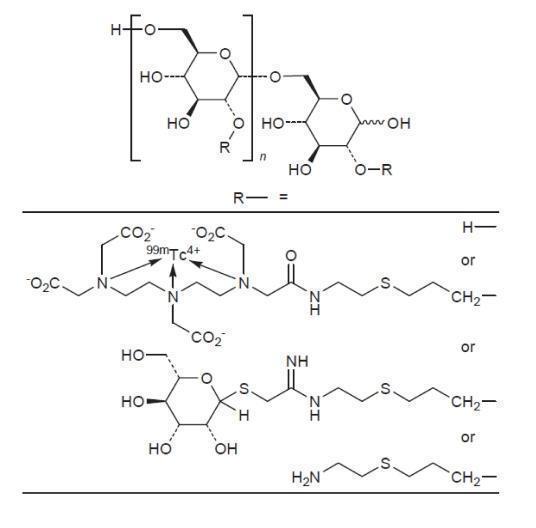
- Chemically, technetium Tc 99m tilmanocept consists of technetium Tc 99m, dextran 3-[(2-aminoethyl)thio]propyl 17-carboxy-10,13,16-tris(carboxymethyl)-8-oxo-4-thia-7,10,13,16-tetraazaheptadec-1-yl 3-[[2-[[1-imino-2-(D-mannopyranosylthio)ethyl]amino]ethyl]thio]propyl ether complexes.
- The molecular formula of technetium Tc 99m tilmanocept is [C
- The calculated average molecular weight of tilmanocept ranges from 15,281 to 23,454 g/mol.
- Technetium Tc 99m tilmanocept has the following structural formula:
- Advise patients to seek medical attention for reactions following injection of Lymphoseek such as difficulty breathing, skin rash, or other allergy manifestations.
- Inform nursing women to pump and discard breast milk for at least 24 hours following administration of Lymphoseek injection

- Five Lymphoseek kit vials (250 mcg tilmanocept per vial) NDC 65857-400-01
- Five vials of DILUENT for Lymphoseek NDC 65857-401-45
- Prescribing information
- Five Radioassay information shield labels
- Twenty-five labels for product vials and individual syringes



