Brand Name
Topicort
Generic Name
Desoximetasone
View Brand Information FDA approval date: January 17, 1985
Classification: Corticosteroid
Form: Spray, Ointment, Cream, Gel
What is Topicort (Desoximetasone)?
Desoximetasone ointment USP.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Topicort (Desoximetasone)
1DESCRIPTION
Topicort
Each gram of Topicort
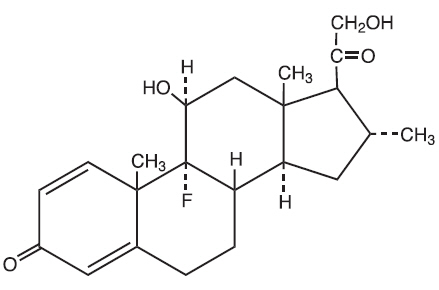
The chemical name of desoximetasone is Pregna-1, 4-diene-3, 20-dione, 9-fluoro-11, 21-dihydroxy- 16-methyl-,(11β,16α)-.
Desoximetasone has the molecular formula C
The structural formula is:
2CLINICAL PHARMACOLOGY
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
2.1Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
Pharmacokinetic studies in men with Topicort
3INDICATIONS AND USAGE
Topicort
4CONTRAINDICATIONS
Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
5WARNINGS
Keep out of reach of children.
6ADVERSE REACTIONS
The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximate decreasing order of occurrence:
Burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae, and miliaria.
In controlled clinical studies the incidence of adverse reactions was low (0.2%) for Topicort
7OVERDOSAGE
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (see
8DOSAGE AND ADMINISTRATION
Apply a thin film of Topicort
9HOW SUPPLIED
Topicort
15 gram tubes (NDC 51672-5263-1), 30 gram tubes (NDC 51672-5263-2), 60 gram tubes (NDC 51672-5263-3) and 100 gram tubes (NDC 51672-5263-7).
10PRINCIPAL DISPLAY PANEL - 15 g Tube Carton
NDC 51672-5263-1
15 g
Rx only
0.05%
OINTMENT
Topicort®
Desoximetasone Ointment USP, 0.05%
FOR TOPICAL USE ONLY. NOT FOR ORAL, OPHTHALMIC, OR INTRAVAGINAL USE.
Desoximetasone Ointment USP, 0.05%
FOR TOPICAL USE ONLY. NOT FOR ORAL, OPHTHALMIC, OR INTRAVAGINAL USE.
Keep this and all medications out of the reach of children.
TaroPharma®
