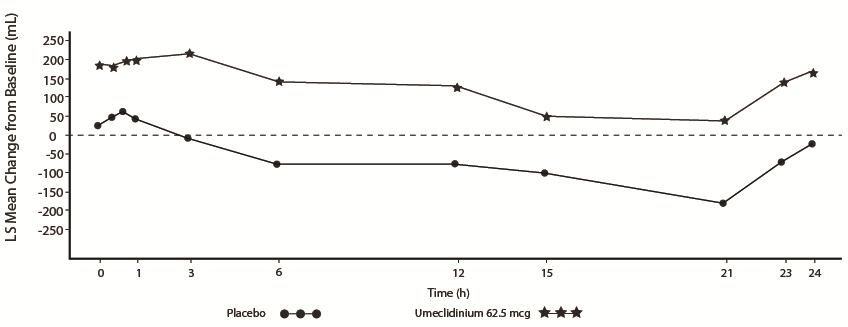
Lung Function
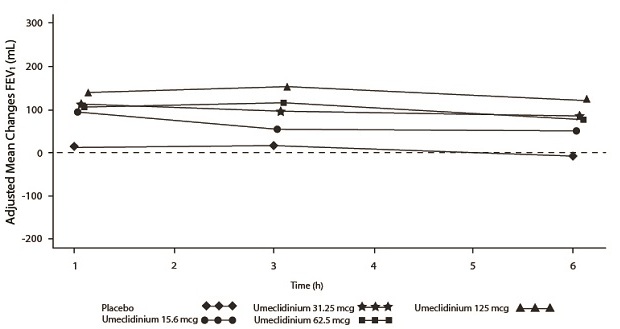
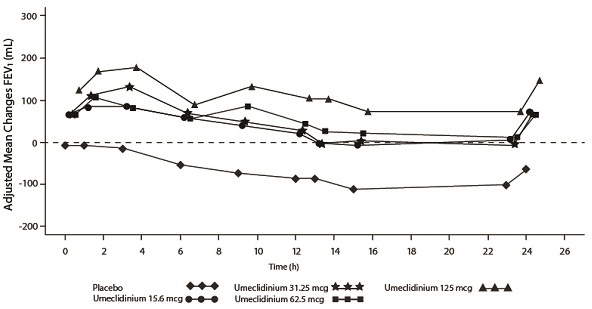
The efficacy of INCRUSE ELLIPTA in combination with an ICS/LABA was evaluated in 4 randomized, double-blind, parallel-group trials in subjects with COPD. These trials, all of similar study design, were of 12-weeks’ treatment duration. Subjects were randomized to INCRUSE ELLIPTA 62.5 mcg + ICS/LABA or placebo + ICS/LABA. Entry criteria for subjects enrolled in these trials were similar to those described above in Section 14.2. The primary endpoint for these trials was change from baseline in trough (predose) FEV
Combination with Fluticasone Furoate + Vilanterol
Trial 4 (NCT01957163) and Trial 5 (NCT02119286) randomized subjects to INCRUSE ELLIPTA 62.5 mcg + FF/VI 100 mcg/25 mcg administered once daily or placebo + FF/VI 100 mcg/25 mcg administered once daily. Trial population demographics and results for Trials 4 and 5 were similar; therefore, only Trial 4 results are presented below.
Subjects in Trial 4 across all treatment arms had a mean age of 64 years and an average smoking history of 50 pack-years, with 42% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV
The primary endpoint was change from baseline in trough (predose) FEV
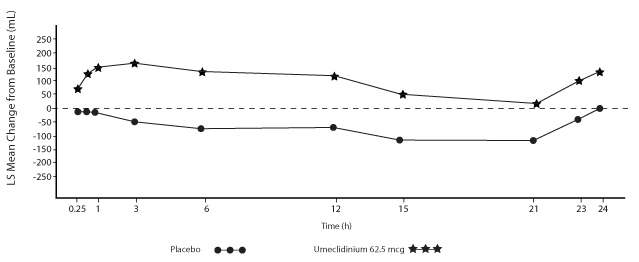
Combination with Fluticasone Propionate + Salmeterol
Trial 6 (NCT01772134) and Trial 7 (NCT01772147) randomized subjects to INCRUSE ELLIPTA 62.5 mcg + FP/SAL 250 mcg/50 mcg or placebo + FP/SAL 250 mcg/50 mcg. The treatments with INCRUSE ELLIPTA and placebo were administered once daily, while the FP/SAL treatment was administered twice daily. Trial population demographics and results for Trials 6 and 7 were similar; therefore, only Trial 6 results are presented below.
Subjects in Trial 6 across all treatment arms had a mean age of 63 years and an average smoking history of 50 pack-years, with 54% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV
The primary endpoint was change from baseline in trough (predose) FEV
Exacerbations
In Trial 8 (NCT02164513), a total of 10,355 subjects with COPD with a history of 1 or more moderate or severe exacerbations in the prior 12 months were randomized (2:2:1) to receive fluticasone furoate/umeclidinium/vilanterol 100 mcg/62.5 mcg/25 mcg (n = 4,151), fluticasone furoate/vilanterol 100 mcg/25 mcg (n = 4,133), or umeclidinium/vilanterol 62.5 mcg/25 mcg (n = 2,070) administered once daily in a 12-month trial. The population demographics across all treatments were: mean age of 65 years, 77% white, 66% male, and an average smoking history of 46.6 pack-years, with 35% identified as current smokers. At trial entry, the most common COPD medications were ICS + anticholinergic + LABA (34%), ICS + LABA (26%), anticholinergic + LABA (8%), and anticholinergic (7%). The mean postbronchodilator percent predicted FEV
The primary endpoint was annual rate of on-treatment moderate and severe exacerbations in subjects treated with fluticasone furoate/umeclidinium/vilanterol compared with the fixed-dose combinations of fluticasone furoate/vilanterol and umeclidinium/vilanterol. Exacerbations were defined as worsening of 2 or more major symptoms (dyspnea, sputum volume, and sputum purulence) or worsening of any 1 major symptom together with any 1 of the following minor symptoms: sore throat, colds (nasal discharge and/or nasal congestion), fever without other cause, and increased cough or wheeze for at least 2 consecutive days. Exacerbations were considered to be of moderate severity if treatment with systemic corticosteroids and/or antibiotics was required and were considered to be severe if resulted in hospitalization or death.
Evidence of efficacy for INCRUSE ELLIPTA on COPD exacerbations was established by the efficacy of the umeclidinium component of fluticasone furoate/umeclidinium/vilanterol in Trial 8. Treatment with fluticasone furoate/umeclidinium/vilanterol statistically significantly reduced the on-treatment annual rate of moderate/severe exacerbations by 15% compared with fluticasone furoate/vilanterol (