Generic Name
methYLphenidate
Brand Names
Relexxii, methYLphenidate Transdermal, Daytrana, Ritalin, Jornay, Quillivant, Metadate, Methylin, QuilliChew, Concerta, Cotempla, Aptensio
FDA approval date: December 31, 1955
Classification: Central Nervous System Stimulant
Form: Patch, Tablet, Suspension, Capsule, Solution
What is Relexxii (methYLphenidate)?
Methylphenidate hydrochloride extended-release capsules are indicated for the treatment of Attention Deficit Hyperactivity Disorder , in pediatric patients 6 to 12 years of age. Methylphenidate hydrochloride extended-release capsule is a central nervous system stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder in pediatric patients 6 to 12 years of age .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
RELEXXII (methylphenidate hydrochloride)
WARNING: ABUSE, MISUSE, AND ADDICTION
RELEXXII has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including RELEXXII, can result in overdose and death
Before prescribing RELEXXII, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout RELEXXII treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction
1INDICATIONS AND USAGE
RELEXXII is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in adults (up to the age of 65 years) and pediatric patients 6 years of age and older
Limitations of Use
The use of RELEXXII is not recommended in pediatric patients younger than 6 years of age because they had higher plasma exposure and a higher incidence of adverse reactions (e.g., weight loss) than patients 6 years and older at the same dosage
2DOSAGE FORMS AND STRENGTHS
RELEXXII (methylphenidate hydrochloride extended-release tablets) are available in the following strengths:
- 18 mg: yellow with “TL706” imprinted in black ink
- 27 mg: gray with “TL707” imprinted in black ink
- 36 mg: white with “TL708” imprinted in black ink
- 45 mg: pink with “TL711” imprinted in black ink
- 54 mg: pink with “TL709” imprinted in black ink
- 63 mg: orange with “TL700” imprinted in black ink and
- 72 mg: blue with “TL710” imprinted in black ink.
3CONTRAINDICATIONS
RELEXXII is contraindicated in patients:
- with a known hypersensitivity to methylphenidate or other components of RELEXXII. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with other methylphenidate products
- receiving concomitant treatment with monoamine oxidase inhibitors (MAOIs), and also within 14 days following discontinuation of treatment with a MAOI, because of the risk of hypertensive crisis
4ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Abuse, Misuse, and Addiction
- Known hypersensitivity to methylphenidate or other ingredients
- Hypertensive crisis when used concomitantly with monoamine oxidase inhibitors
- Risks to Patients with Serious Cardiac Disease
- Increased Blood Pressure and Heart Rate
- Psychiatric Adverse Reactions
- Priapism
- Peripheral Vasculopathy, including Raynaud’s Phenomenon
- Long-Term Suppression of Growth in Pediatric Patients
- Potential for Gastrointestinal Obstruction
- Acute Angle Closure Glaucoma
- Increased Intraocular Pressure and Glaucoma
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of RELEXXII for the treatment of ADHD is based on adequate and well-controlled studies of another formulation of methylphenidate hydrochloride extended-release tablets. Below is a display of adverse reactions from those adequate and well-controlled studies in ADHD.
Adults and pediatric patients 6 to 17 years with ADHD were evaluated in six controlled clinical studies and eleven open-label clinical studies (see Table 3). Safety was assessed by collecting adverse reactions, vital signs, weights, and electrocardiograms (ECGs), and by performing physical examinations and laboratory analyses. A total of 3,906 patients participated in the clinical trials.
Table 3: Exposure in Double-Blind and Open-Label Clinical Studies of Another Formulation of Methylphenidate Hydrochloride Extended-Release Tablets
* 108 mg is 1.5 times the maximum recommended dosage of RELEXXII.
The most common adverse reactions in double-blind clinical trials (>5%) were:
- Pediatric patients 6 to 17 years: abdominal pain upper (see Table 4).
- Adults: decreased appetite, headache, dry mouth, nausea, insomnia, anxiety, dizziness, weight decreased, irritability, and hyperhidrosis (see Table 5).
The most common adverse reactions associated with discontinuation (≥1%) from either pediatric or adult clinical trials were anxiety, irritability, insomnia, and blood pressure increased
Adverse reactions in either the pediatric or adult double-blind adverse reactions tables may be relevant for both patient populations.
Pediatric Patients 6 to 17 Years
Table 4 lists the adverse reactions reported in 1% or more of another formulation of methylphenidate hydrochloride extended-release tablet-treated pediatric patients (6 to 17 years) in four placebo-controlled, double-blind clinical trials.
Table 4: Adverse Reactions Reported by ≥1% of Pediatric Patients (6 to 17 years) Treated with Another Formulation of Methylphenidate Hydrochloride Extended-release Tablets in Four Placebo-Controlled, Double-Blind Clinical Trials
Adults
Table 5 lists the adverse reactions reported in 1% or more of adults treated with another formulation of methylphenidate hydrochloride extended-release tablets in two placebo-controlled, double-blind clinical trials.
Table 5: Adverse Reactions Reported by ≥1% of Adults Treated with Another Formulation of Methylphenidate Hydrochloride Extended-release Tablets in Two Placebo-Controlled, Double-Blind Clinical Trials
Adverse Reactions Observed in Clinical Trials with Another Formulation of Methylphenidate Hydrochloride Extended-release Tablets
This section includes adverse reactions reported with use of another formulation of methylphenidate hydrochloride extended-release tablets in double-blind trials that do not meet the criteria specified for Table 4 or Table 5 and all adverse reactions reported by the other formulation of methylphenidate hydrochloride extended-release tablets-treated patients who participated in open-label and postmarketing clinical trials.
Blood and Lymphatic System Disorders: Leukopenia
Eye Disorders: Accommodation disorder, Dry eye
Vascular Disorders: Hot flush
Gastrointestinal Disorders: Abdominal discomfort, Abdominal pain, Diarrhea
General Disorders and Administrative Site Conditions: Asthenia, Fatigue, Feeling jittery, Thirst
Infections and Infestations: Sinusitis
Investigations: Alanine aminotransferase increased, Blood pressure increased, Cardiac murmur, Heart rate increased
Musculoskeletal and Connective Tissue Disorders: Muscle spasms
Nervous System Disorders: Lethargy, Psychomotor hyperactivity, Somnolence
Psychiatric Disorders: Anger, Hypervigilance, Mood altered, Mood swings, Panic attack, Sleep disorder, Tearfulness, Tic
Reproductive System and Breast Disorders: Erectile dysfunction
Respiratory, Thoracic and Mediastinal Disorders: Dyspnea
Skin and Subcutaneous Tissue Disorders: Rash, Rash macular
Vascular Disorders: Hypertension
Discontinuation Due to Adverse Reactions
Adverse reactions in the four placebo-controlled studies of pediatric patients (6 to 17 years) leading to discontinuation occurred in 2 patients (0.6%) treated with another formulation of methylphenidate hydrochloride extended-release tablets including depressed mood (1, 0.3%) and headache and insomnia (1, 0.3%), and 6 placebo patients (1.9%) including headache and insomnia (1, 0.3%), irritability (2, 0.6%), headache (1, 0.3%), psychomotor hyperactivity (1, 0.3%), and tic (1, 0.3%).
In the two placebo-controlled studies of adults, 25 patients (6.0%) treated with another formulation of methylphenidate hydrochloride extended-release tablets and 6 placebo patients (2.8%) discontinued due to an adverse reaction. Incidence of >0.5% in patients treated with another formulation of methylphenidate hydrochloride extended-release tablets included anxiety (1.7%), irritability (1.4%), blood pressure increased (1.0%), and nervousness (0.7%). In placebo patients, blood pressure increased and depressed mood had an incidence of >0.5% (0.9%).
In the eleven open-label studies of pediatric patients and adults, 266 patients (7.0%) treated with another formulation of methylphenidate hydrochloride extended-release tablets discontinued due to an adverse reaction. Incidence of >0.5% included insomnia (1.2%), irritability (0.8%), anxiety (0.7%), decreased appetite (0.7%), and tic (0.6%).
Tics
In a long-term uncontrolled study (n=432 pediatric patients 6 to 12 years), the cumulative incidence of new onset of tics was 9% after 27 months of treatment with another formulation of methylphenidate hydrochloride extended-release tablets.
In a second uncontrolled study (n=682 pediatric patients 6 to 12 years) the cumulative incidence of new-onset tics was 1% (9/682). The treatment period was up to 9 months with mean treatment duration of 7.2 months.
Blood Pressure and Heart Rate Increases
In the laboratory classroom clinical trials in pediatric patients 6 to 12 years (Studies 1 and 2), both another formulation of methylphenidate hydrochloride extended-release tablets once daily and methylphenidate three times daily increased resting pulse by an average of 2 to 6 bpm and produced average increases of systolic and diastolic blood pressure of roughly 1 to 4 mm Hg during the day, relative to placebo. In the placebo-controlled trial in pediatric patients 13 to 17 years (Study 4), mean increases from baseline in resting pulse rate were observed with another formulation of methylphenidate hydrochloride extended-release tablets and placebo at the end of the double-blind phase (5 and 3 beats/minute, respectively). Mean increases from baseline in blood pressure at the end of the double-blind phase for another formulation of methylphenidate hydrochloride extended-release tablets and placebo-treated patients were 0.7 and 0.7 mm Hg (systolic) and 2.6 and 1.4 mm Hg (diastolic), respectively. In one placebo-controlled study in adults (Study 6), dose-dependent mean increases of 3.9 to 9.8 bpm from baseline in standing pulse rate were observed with another formulation of methylphenidate hydrochloride extended-release tablets at the end of the double-blind treatment vs. an increase of 2.7 beats/minute with placebo. Mean changes from baseline in standing blood pressure at the end of double-blind treatment ranged from 0.1 to 2.2 mm Hg (systolic) and -0.7 to 2.2 mm Hg (diastolic) for another formulation of methylphenidate hydrochloride extended-release tablets and was 1.1 mm Hg (systolic) and -1.8 mm Hg (diastolic) for placebo. In a second placebo-controlled study in adults (Study 5), mean changes from baseline in resting pulse rate were observed for another formulation of methylphenidate hydrochloride extended-release tablets and placebo at the end of the double-blind treatment (3.6 and –1.6 beats/minute, respectively). Mean changes from baseline in blood pressure at the end of the double–blind treatment for another formulation of methylphenidate hydrochloride extended-release tablets and placebo-treated patients were –1.2 and –0.5 mm Hg (systolic) and 1.1 and 0.4 mm Hg (diastolic), respectively
4.2Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of another formulation of methylphenidate hydrochloride extended-release tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Pancytopenia, Thrombocytopenia, Thrombocytopenic purpura
Cardiac Disorders: Angina pectoris, Bradycardia, Extrasystoles, Supraventricular tachycardia, Ventricular extrasystoles
Eye Disorders: Diplopia, Increased intraocular pressure, Mydriasis, Visual impairment
General Disorders: Chest pain, Chest discomfort, Drug effect decreased, Hyperpyrexia, Therapeutic response decreased
Hepatobiliary Disorders: Hepatocellular injury, Acute hepatic failure
Immune System Disorders: Hypersensitivity reactions such as Angioedema, Anaphylactic reactions, Auricular swelling, Bullous conditions, Exfoliative conditions, Urticarias, Pruritus NEC, Rashes, Eruptions, and Exanthemas NEC
Investigations: Blood alkaline phosphatase increased, Blood bilirubin increased, Hepatic enzyme increased, Platelet count decreased, White blood cell count abnormal
Musculoskeletal, Connective Tissue and Bone Disorders: Arthralgia, Myalgia, Muscle twitching, Rhabdomyolysis
Nervous System Disorders: Convulsion, Grand mal convulsion, Dyskinesia, Serotonin syndrome in combination with serotonergic drugs, Motor and Verbal Tics
Psychiatric Disorders: Disorientation, Hallucination, Hallucination auditory, Hallucination visual, Mania, Logorrhea, Libido changes
Reproductive System and Breast Disorders: Priapism
Skin and Subcutaneous Tissue Disorders: Alopecia, Erythema
Vascular Disorders: Raynaud's phenomenon
5OVERDOSAGE
Clinical Effects of Overdose
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Overdose Management
Consider the possibility of multiple drug ingestion. The pharmacokinetic profile of RELEXXII should be considered when treating patients with overdose. Because methylphenidate has a large volume of distribution and is rapidly metabolized, dialysis is not useful. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
6DESCRIPTION
RELEXXII contains methylphenidate, a CNS stimulant, present as methylphenidate hydrochloride salt. Chemically, methylphenidate hydrochloride is d,l (racemic) methyl α-phenyl-2-piperidineacetate hydrochloride. Its empirical formula is C
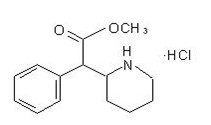
Methylphenidate hydrochloride is a white, odorless crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. It has a pKa of 8.71 (at 21.5°C). Its molecular weight is 269.77.
RELEXXII is for oral administration and is available in the following strengths: 18 mg, 27 mg, 36 mg, 45 mg, 54 mg, 63 mg, and 72 mg containing methylphenidate hydrochloride (equivalent to 15.6 mg, 23.4 mg, 31.1 mg, 38.9 mg, 46.7 mg, 54.5 mg, and 62.3 mg methylphenidate respectively).
RELEXXII contains the following inactive ingredients: cellulose acetate, colloidal silicon dioxide, ferrosoferric oxide, hypromellose, iron oxide black, lactose monohydrate, magnesium stearate, phosphoric acid, polyethylene glycol, polyethylene oxide, sodium chloride, succinic acid, titanium dioxide, triacetin.
RELEXXII also contains the following color additives:
27 mg: FD&C Yellow #6 Aluminum Lake, FD&C Blue #2 Aluminum Lake, FD&C Red #40 Aluminum Lake
45 mg: FD&C Red #40 Aluminum Lake
54 mg: FD&C Yellow #6 Aluminum Lake, FD&C Red #40 Aluminum Lake, FD&C Blue #2 Aluminum Lake
18 and 63 mg: iron oxide red, iron oxide yellow
72 mg: FD&C Blue #1 Aluminum Lake
System Components and Performance
RELEXXII uses osmotic pressure to deliver methylphenidate hydrochloride at a controlled rate. The system, which resembles a conventional tablet in appearance, comprises an osmotically active bilayer core surrounded by a semipermeable membrane with an immediate-release drug overcoat. The bilayer core is composed of a drug layer containing the drug and excipients, and a push layer containing osmotically active components. There is a precision-laser drilled orifice on the drug-layer end of the tablet. In an aqueous environment, such as the gastrointestinal tract, the drug overcoat dissolves within one hour, providing an initial dose of methylphenidate. Water permeates through the membrane into the tablet core. As the osmotically active polymer excipients expand, methylphenidate is released through the orifice. The membrane controls the rate at which water enters the tablet core, which in turn controls drug delivery. Furthermore, the drug release rate from the system increases with time over a period of 6 to 7 hours due to the drug-concentration gradient incorporated into the two drug layers of core of RELEXXII. The biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the stool as a tablet shell along with insoluble core components. It is possible that RELEXXII may be visible on abdominal x-rays under certain circumstances, especially when digital enhancing techniques are utilized.
7CLINICAL STUDIES
The efficacy of RELEXXII for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in pediatric patients (6 to 17 years) and adult patients is based upon adequate and well-controlled studies of another formulation of methylphenidate hydrochloride extended-release tablets (referred to as “methylphenidate hydrochloride extended-release tablets” in the section below). The results of these adequate and well-controlled studies are presented below.
Methylphenidate hydrochloride extended-release tablets was demonstrated to be effective in the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in four randomized, double-blind, placebo-controlled studies in pediatric patients 6 to 17 years and two double-blind placebo-controlled studies in adults who met the Diagnostic and Statistical Manual 4
Pediatric Patients 6 to 12 Years
Three double-blind, active- and placebo-controlled studies were conducted in 416 pediatric patients 6 to 12 years. The controlled studies compared methylphenidate hydrochloride extended-release tablets given once daily (18 mg, 36 mg, or 54 mg), methylphenidate given three times daily over 12 hours (15 mg, 30 mg, or 45 mg total daily dose), and placebo in two single-center, 3-week crossover studies (Studies 1 and 2) and in a multicenter, 4-week, parallel-group comparison (Study 3). The primary comparison of interest in all three trials was methylphenidate hydrochloride extended-release tablets versus placebo.
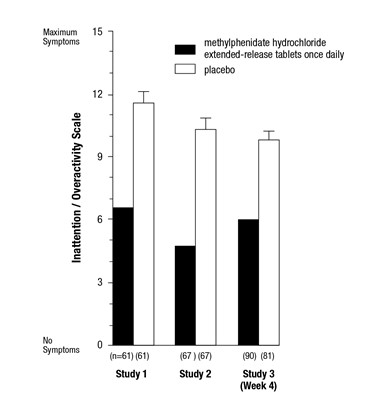
Symptoms of ADHD were evaluated by community schoolteachers using the Inattention/Overactivity with Aggression (IOWA) Conners scale. Statistically significant reduction in the Inattention/Overactivity subscale versus placebo was shown consistently across all three controlled studies for methylphenidate hydrochloride extended-release tablets. Studies 1 and 2 involved a 3-way crossover of 1 week per treatment arm. Study 3 involved 4 weeks of parallel-group treatments with a Last Observation Carried Forward analysis at Week 4.
The scores for methylphenidate hydrochloride extended-release tablets and placebo for the three studies are presented in Figure 2. Error bars represent the mean plus standard error of the mean.
Figure 2: Mean Community School Teacher IOWA Conners Inattention/Overactivity Scores with Methylphenidate Hydrochloride Extended-Release Tablets Once Daily (18 mg, 36 mg, or 54 mg) and Placebo (Studies 1, 2, and 3)
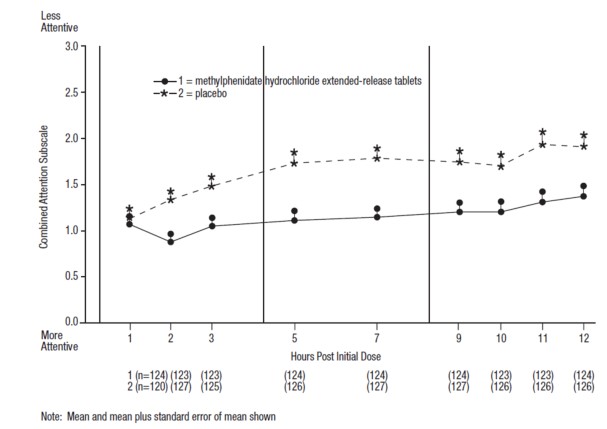
In Studies 1 and 2, symptoms of ADHD were evaluated by laboratory schoolteachers using the Swanson, Kotkin, Agler, M-Fynn, and Pelham (SKAMP) laboratory school rating scale. The combined results from these two studies demonstrated statistically significant improvements in attention and behavior in patients treated with methylphenidate hydrochloride extended-release tablets versus placebo that were maintained through 12 hours after dosing. Figure 3 presents the laboratory schoolteacher SKAMP ratings for methylphenidate hydrochloride extended-release tablets and placebo.
Figure 3: Laboratory School Teacher SKAMP Ratings: Mean (SEM) of Combined Attention (Studies 1 and 2)
Pediatric Patients 13 to 17 years
In a randomized, double-blind, multicenter, placebo-controlled trial (Study 4) involving 177 patients, methylphenidate hydrochloride extended-release tablets was demonstrated to be effective in the treatment of ADHD in pediatric patients aged 13 to 18 years at doses up to 72 mg once daily (1.4 mg/kg/day). Of 220 patients who entered an open 4-week titration phase, 177 were titrated to an individualized dose (maximum of 72 mg once daily) based on meeting specific improvement criteria on the ADHD Rating Scale and the Global Assessment of Effectiveness with acceptable tolerability. Patients who met these criteria were then randomized to receive either their individualized dose of methylphenidate hydrochloride extended-release tablets (18 mg to 72 mg once daily, n=87) or placebo (n=90) during a two-week double-blind phase. At the end of this phase, mean scores for the investigator rating on the ADHD Rating Scale demonstrated that methylphenidate hydrochloride extended-release tablets was statistically significantly superior to placebo.
Adults
Two double-blind, placebo-controlled studies were conducted in 627 adults aged 18 to 65 years. The controlled studies compared methylphenidate hydrochloride extended-release tablets administered once daily and placebo in a multicenter, parallel-group, 7-week dose-titration study (Study 5) (36 mg to 108 mg once daily) and in a multicenter, parallel-group, 5-week, fixed-dose study (Study 6) (18 mg, 36 mg, and 72 mg once daily).
Study 5 demonstrated the effectiveness of methylphenidate hydrochloride extended-release tablets in the treatment of ADHD in adults aged 18 to 65 years at doses from 36 mg once daily to 108 mg once daily based on the change from baseline to final study visit on the Adult ADHD Investigator Rating Scale (AISRS). Of 226 patients who entered the 7-week trial, 110 were randomized to methylphenidate hydrochloride extended-release tablets and 116 were randomized to placebo. Treatment was initiated at 36 mg once daily and patients continued with incremental increases of 18 mg once daily (36 mg to 108 mg once daily) based on meeting specific improvement criteria with acceptable tolerability. At the final study visit, mean change scores (LS Mean, SEM) for the investigator rating on the AISRS demonstrated methylphenidate hydrochloride extended-release tablets was statistically significantly superior to placebo.
Study 6 was a multicenter, double-blind, randomized, placebo-controlled, parallel-group, dose-response study (5-week duration) with 3 fixed-dose groups (18 mg, 36 mg, and 72 mg). Patients were randomized to receive methylphenidate hydrochloride extended-release tablets administered at doses of 18 mg (n=101), 36 mg (n=102), 72 mg once daily (n=102), or placebo (n=96). All three doses of methylphenidate hydrochloride extended-release tablets were statistically significantly more effective than placebo in improving CAARS (Conners' Adult ADHD Rating Scale) total scores at double-blind end point in adult subjects with ADHD.
8HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
RELEXXII
- 18 mg yellow tablets with "TL706" imprinted in black ink
- 27 mg gray tablets with "TL707" imprinted in black ink
- 36 mg white tablets with "TL708" imprinted in black ink
- 45 mg pink tablets with "TL711" imprinted in black ink
- 54 mg pink tablets with "TL709" imprinted in black ink
- 63 mg orange tablets with "TL700" imprinted in black ink
- 72 mg blue tablets with "TL710" imprinted in black ink
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from humidity.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Abuse, Misuse, and Addiction
Educate patients and their families about the risks of abuse, misuse, and addiction of RELEXXII, which can lead to overdose and death, and proper disposal of any unused drug
Administration Instructions
Instruct patients to swallow RELEXXII whole with the aid of liquids, and not to chew, divide, or crush the tablets. Advise patients that the medication is contained within a nonabsorbable shell designed to release the drug at a controlled rate. The tablet shell, along with insoluble core components, is eliminated from the body; advise patients not to be concerned if they occasionally notice in their stool something that looks like a tablet
Risks to Patients with Serious Cardiac Disease
Advise patients that there are potential risks to patients with serious cardiac disease, including sudden death with RELEXXII use. Instruct patients to contact a healthcare provider immediately if they develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease
Increased Blood Pressure and Heart Rate
Advise patients and their caregivers that RELEXXII can cause elevations of their blood pressure and pulse rate
Psychiatric Adverse Reactions
Advise patients and their caregivers that RELEXXII, at recommended doses, can cause psychotic or manic symptoms, even in patients without prior history of psychotic symptoms or mania
Priapism
Advise patients, caregivers, and family members of the possibility of painful or prolonged penile erections (priapism). Instruct the patient to seek immediate medical attention in the event of priapism
Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, including Raynaud’s Phenomenon]
- Instruct patients beginning treatment with RELEXXII about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their healthcare provider any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking RELEXXII.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Long-Term Suppression of Growth in Pediatric Patients
Advise patients, families and caregivers that RELEXXII may cause slowing of growth and weight loss
Increased Intraocular Pressure (IOP) and Glaucoma
Advise patients that IOP and glaucoma may occur during treatment with RELEXXII
Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
Advise patients that motor and verbal tics and worsening of Tourette’s Syndrome may occur during treatment with RELEXXII. Instruct patients to notify their healthcare provider if emergence of new tics or worsening of tics or Tourette’s syndrome occurs
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to RELEXXII during pregnancy
Manufactured for:
Vertical Pharmaceuticals, LLC
Alpharetta, GA 30005
1-800-444-5164
www.verticalpharma.com
200438-8
Patent numbers:
US 9,855,258
US 9,827,234
US 9,707,217
US 10,265,308
US 10,695,336

10PRINCIPAL DISPLAY PANEL
RELEXXII
NDC 68025-095-10

11PRINCIPAL DISPLAY PANEL
RELEXXII
NDC 68025-095-30

12PRINCIPAL DISPLAY PANEL
RELEXXII
NDC 68025-096-10

13PRINCIPAL DISPLAY PANEL
RELEXXII
NDC 68025-096-30

14PRINCIPAL DISPLAY PANEL
RELEXXII
NDC 68025-097-10

15PRINCIPLE DISPLAY PANEL
RELEXXII
NDC 68025-097-30

16PRINCIPAL DISPLAY PANEL
RELEXXII
NDC 68025-088-30

17PRINCIPAL DISPLAY PANEL
RELEXXII
NDC 68025-098-10

18PRINCIPLE DISPLAY PANEL
RELEXXII
NDC 68025-098-30

19PRINCIPAL DISPLAY PANEL
RELEXXII
NDC 68025-089-30

20PRINCIPAL DISPLAY PANEL
RELEXXII
NDC 68025-084-30
