Generic Name
Sotalol
Brand Names
Betapace, Sotylize
FDA approval date: May 04, 2000
Classification: Antiarrhythmic
Form: Injection, Tablet, Solution
What is Betapace (Sotalol)?
Sotalol hydrochloride tablets are an antiarrhythmic indicated for: the treatment of life threatening ventricular arrhythmias.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
BETAPACE (sotalol hydrochloride)
WARNING: LIFE-THREATENING PROARRHYTHMIA
To minimize the risk of drug-induced arrhythmia, initiate or reinitiate oral sotalol in a facility that can provide cardiac resuscitation and continuous electrocardiographic monitoring.
Sotalol can cause life-threatening ventricular tachycardia associated with QT interval prolongation.
If the QT interval prolongs to 500 msec or greater, reduce the dose, lengthen the dosing interval, or discontinue the drug.
Calculate creatinine clearance to determine appropriate dosing
1DOSAGE FORMS AND STRENGTHS
Betapace is supplied as capsule-shaped, light-blue, scored tablets:
- 80 mg imprinted with “BETAPACE” on one side and 80 mg on the other
- 120 mg imprinted with “BETAPACE” on one side and 120 mg on the other
- 160 mg imprinted with “BETAPACE” on one side and 160 mg on the other
Betapace AF is supplied as capsule-shaped, white scored tablet:
- 80 mg imprinted with “BHCP” on one side and 80 mg on the other
- 120 mg imprinted with “BHCP” on one side and 120 mg on the other
- 160 mg imprinted with “BHCP” on one side and 160 mg on the other
2CONTRAINDICATIONS
Betapace/Betapace AF is contraindicated in patients with:
- Sinus bradycardia, sick sinus syndrome, second and third degree AV block, unless a functioning pacemaker is present
- Congenital or acquired long QT syndromes
- Cardiogenic shock or decompensated heart failure
- Serum potassium <4 mEq/L
- Bronchial asthma or related bronchospastic conditions
- Hypersensitivity to sotalol
For the treatment of AFIB/AFL, Betapace/Betapace AF is also contraindicated in patients with:
- Baseline QT interval >450 msec
3OVERDOSAGE
Intentional or accidental overdosage with sotalol has resulted in death.
Symptoms and Treatment of Overdosage
The most common signs to be expected are bradycardia, congestive heart failure, hypotension, bronchospasm, and hypoglycemia. In cases of massive intentional overdosage (2 to 16 grams) of sotalol the following clinical findings were seen: hypotension, bradycardia, cardiac asystole, prolongation of QT interval, Torsade de Pointes, ventricular tachycardia, and premature ventricular complexes. If overdosage occurs, therapy with sotalol should be discontinued and the patient observed closely. Because of the lack of protein binding, hemodialysis is useful for reducing sotalol plasma concentrations. Patients should be carefully observed until QT intervals are normalized and the heart rate returns to levels >50 bpm.
The occurrence of hypotension following an overdose may be associated with an initial slow drug elimination phase (half-life of 30 hours) thought to be due to a temporary reduction of renal function caused by the hypotension. In addition, if required, the following therapeutic measures are suggested:
Bradycardia or Cardiac Asystole: Atropine, another anticholinergic drug, a beta-adrenergic agonist, or transvenous cardiac pacing.
Heart Block: (second and third degree) transvenous cardiac pacemaker.
Hypotension: (depending on associated factors) epinephrine rather than isoproterenol or norepinephrine may be useful.
Bronchospasm: Aminophylline or aerosol beta-2-receptor stimulant. Higher than normal doses of beta-2-receptor stimulants may be required.
Torsade de Pointes: DC cardioversion, transvenous cardiac pacing, epinephrine, magnesium sulfate.
4DESCRIPTION
Betapace/Betapace AF contains sotalol hydrochloride, an antiarrhythmic drug with Class II (beta-adrenoreceptor blocking) and Class III (cardiac action potential duration prolongation) properties. Betapace is supplied as a light-blue, capsule-shaped tablet for oral administration. Betapace AF is supplied as a white, capsule-shaped tablet for oral administration. Sotalol hydrochloride is a white, crystalline solid with a molecular weight of 308.8. It is hydrophilic, soluble in water, propylene glycol, and ethanol, but is only slightly soluble in chloroform. Chemically, sotalol hydrochloride is d,l-
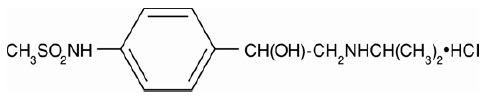
Betapace Tablets contain the following inactive ingredients: microcrystalline cellulose, lactose, starch, stearic acid, magnesium stearate, colloidal silicon dioxide, and FD&C blue color #2 (aluminum lake, conc.).
Betapace AF Tablets contain the following inactive ingredients: microcrystalline cellulose, lactose, starch, stearic acid, magnesium stearate, and colloidal silicon dioxide.
5HOW SUPPLIED/STORAGE AND HANDLING
Betapace (sotalol hydrochloride); capsule-shaped light-blue scored tablets, imprinted with the strength and “BETAPACE,” are available as follows:
NDC 83107-005-10 80 mg strength, bottle of 100
Betapace AF (sotalol hydrochloride); capsule-shaped white scored tablets, imprinted with the strength and “BHCP” are available as follows:
NDC 83107-008-60 80 mg strength, bottle of 60
Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F)
6PATIENT COUNSELING INFORMATION
- Advise patients to contact their healthcare provider in the event of syncope, pre-syncopal symptoms or cardiac palpitations.
- Advise patients that their electrolytes and ECG will be monitored during treatment
- Advise patients to contact their healthcare provider in the event of conditions that could lead to electrolyte changes such as severe diarrhea, unusual sweating, vomiting, less appetite than normal, or excessive thirst
- Advise patients not to change the Betapace/Betapace AF dose prescribed by their healthcare provider.
- Advise patients that they should not miss a dose, but if they do miss a dose they should not double the next dose to compensate for the missed dose: they should take the next dose at the regularly scheduled time
- Advise patients to not interrupt or discontinue Betapace/Betapace AF without their physician’s advice, that they should get their prescription for sotalol filled and refilled on time, so they do not interrupt treatment
- Advise patients to not start taking other medications without first discussing new medications with their healthcare provider.
- Advise patients to avoid taking Betapace/Betapace AF within two hours of taking antacids that contain aluminum oxide or magnesium hydroxide
- Inform patients or caregivers that there is a risk of hypoglycemia when Betapace/Betapace AF is given to patients who are fasting or who are vomiting. Inform patients to notify their healthcare provider if they experience symptoms of hypoglycemia.
Lactation
- Advise women not to breastfeed while on treatment with Betapace
©2023, Legacy Pharma Inc. All rights reserved.
Manufactured for:

Legacy Pharma Inc.
7PRINCIPAL DISPLAY PANEL - 80 mg BETAPACE

NDC 83107-005-10
Betapace
80 mg
Rx only
LEGACY
Each tablet contains
Dosage: See package
Store at 25°C (77°F);
Dispense in a
Mfd for:
Made in Spain
Rev. 8/24
PRINCIPAL DISPLAY PANEL - 120 mg BETAPACE

NDC 83107-006-10
Betapace
120 mg
Rx only
LEGACY
Each tablet
Dosage: See
Store at 25°C
Dispense in a
Mfd for:
Made in Spain
Rev. 8/24
PRINCIPAL DISPLAY PANEL - 160 mg BETAPACE

NDC 83107-007-10
Betapace
160 mg
Rx only
LEGACY
Each tablet
Dosage: See
Store at 25°C
Dispense in a
Mfd for:
Made in Spain
Rev. 8/24
PRINCIPAL DISPLAY PANEL - 80 mg BETAPACE AF

NDC 83107-008-60
BETAPACE AF®
(sotalol HCl)
Patient Pack
(sotalol HCl)
Patient Pack
80 mg
Rx only
LEGACY
Each tablet
Dosage: Take as
Please see
Store at 25°C
Mfd for:
Made in Spain
Rev. 8/24
PRINCIPAL DISPLAY PANEL - 120 mg BETAPACE AF

NDC 83107-009-60
BETAPACE AF®
(sotalol HCl)
Patient Pack
(sotalol HCl)
Patient Pack
120 mg
Rx only
LEGACY
Each tablet
Dosage: Take as
Please see
Store at 25°C
Mfd for:
Made in Spain
Rev. 8/24
PRINCIPAL DISPLAY PANEL - 160 mg BETAPACE AF

NDC 83107-010-60
BETAPACE AF®
(sotalol HCl)
Patient Pack
(sotalol HCl)
Patient Pack
160 mg
Rx only
LEGACY
Each tablet
Dosage: Take as
Please see
Store at 25°C
Mfd for:
Made in Spain
Rev. 8/24