Generic Name
Tamsulosin
Brand Names
Dutasteride, Flomax
FDA approval date: September 12, 1997
Classification: alpha-Adrenergic Blocker
Form: Capsule
What is Dutasteride (Tamsulosin)?
Tamsulosin hydrochloride capsules are indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia . Tamsulosin hydrochloride capsules are not indicated for the treatment of hypertension. Tamsulosin hydrochloride is an alpha 1 adrenoceptor antagonist indicated for treatment of the signs and symptoms of benign prostatic hyperplasia , Tamsulosin hydrochloride capsules are not indicated for the treatment of hypertension
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Dutasteride and Tamsulosin Hydrochloride (Dutasteride and Tamsulosin Hydrochloride Capsules)
1DOSAGE AND ADMINISTRATION
The recommended dosage of dutasteride and tamsulosin hydrochloride capsules is 1 capsule (0.5 mg dutasteride and 0.4 mg tamsulosin hydrochloride) taken once daily approximately 30 minutes after the same meal each day.
The capsules should be swallowed whole and not chewed or opened. Contact with the contents of the dutasteride and tamsulosin hydrochloride capsule may result in irritation of the oropharyngeal mucosa.
2DOSAGE FORMS AND STRENGTHS
Dutasteride and tamsulosin hydrochloride capsules, containing 0.5 mg dutasteride and 0.4 mg tamsulosin hydrochloride, are capsules with blue opaque cap imprinted with “C280” and white opaque body imprinted with “0.5/0.4” in black ink containing white to off-white spherical shaped pellets and one oblong, opaque yellow softgel capsule printed with “C300” in black ink.
3CONTRAINDICATIONS
Dutasteride and tamsulosin hydrochloride capsules are contraindicated for use in:
- Pregnancy. Dutasteride use is contraindicated in females who are pregnant. In animal reproduction and developmental toxicity studies, dutasteride inhibited development of male fetus external genitalia. Therefore, dutasteride and tamsulosin hydrochloride capsules may cause fetal harm when administered to a pregnant female.
- Patients with previously demonstrated, clinically significant hypersensitivity (e.g., serious skin reactions, angioedema, urticaria, pruritus, respiratory symptoms) to dutasteride, other 5-alpha-reductase inhibitors, tamsulosin, or any other component of dutasteride and tamsulosin hydrochloride capsules
4DRUG INTERACTIONS
There have been no drug interaction trials using dutasteride and tamsulosin hydrochloride capsules. The following sections reflect information available for the individual components.
4.1Cytochrome P450 Inhibition
Dutasteride
Dutasteride is extensively metabolized in humans by the CYP3A4 and CYP3A5 isoenzymes. The effect of potent CYP3A4 inhibitors on dutasteride has not been studied. Because of the potential for drug-drug interactions, use caution when prescribing a dutasteride-containing product, including dutasteride and tamsulosin hydrochloride capsules, to patients taking potent, chronic CYP3A4 enzyme inhibitors (e.g., ritonavir) [seeClinical Pharmacology ( ].
Tamsulosin
Strong and Moderate Inhibitors of CYP3A4 or CYP2D6:Tamsulosin is extensively metabolized, mainly by CYP3A4 or CYP2D6.
Concomitant treatment with ketoconazole (a strong inhibitor of CYP3A4) resulted in increases in the C
Cimetidine: Treatment with cimetidine resulted in a moderate increase in tamsulosin hydrochloride AUC (44%) [seeWarnings and Precautions ( ].
4.2Warfarin
Dutasteride
Concomitant administration of dutasteride 0.5 mg/day for 3 weeks with warfarin does not alter the steady-state pharmacokinetics of the S- or R-warfarin isomers or alter the effect of warfarin on prothrombin time [seeClinical Pharmacology ( ].
Tamsulosin
A definitive drug-drug interaction trial between tamsulosin hydrochloride and warfarin was not conducted. Results from limited in vitroand in vivostudies are inconclusive. Caution should be exercised with concomitant administration of warfarin and tamsulosin-containing products, including dutasteride and tamsulosin hydrochloride capsules [seeWarnings and Precautions ( ].
4.3Nifedipine, Atenolol, Enalapril
Tamsulosin
Dosage adjustments are not necessary when tamsulosin is administered concomitantly with nifedipine, atenolol, or enalapril
4.4Digoxin and Theophylline
Dutasteride
Dutasteride does not alter the steady-state pharmacokinetics of digoxin when administered concomitantly at a dose of 0.5 mg/day for 3 weeks [seeClinical Pharmacology ( )].
Tamsulosin
Dosage adjustments are not necessary when tamsulosin is administered concomitantly with digoxin or theophylline [seeClinical Pharmacology ( ].
4.5Furosemide
Tamsulosin
Tamsulosin had no effect on the pharmacodynamics (excretion of electrolytes) of furosemide. While furosemide produced an 11% to 12% reduction in tamsulosin hydrochloride C maxand AUC, these changes are expected to be clinically insignificant and do not require adjustment of the dose of tamsulosin [seeClinical Pharmacology ( ].
4.6Calcium Channel Antagonists
Dutasteride
Coadministration of verapamil or diltiazem decreases dutasteride clearance and leads to increased exposure to dutasteride. The change in dutasteride exposure is not considered to be clinically significant. No dosage adjustment of dutasteride is recommended [seeClinical Pharmacology ( ].
4.7Cholestyramine
Dutasteride
Administration of a single 5 mg dose of dutasteride followed 1 hour later by a 12 g dose of cholestyramine does not affect the relative bioavailability of dutasteride [seeClinical Pharmacology ( ].
5OVERDOSAGE
No data are available with regard to overdosage with dutasteride and tamsulosin hydrochloride capsules. The following text reflects information available for the individual components.
Dutasteride
In volunteer trials, single doses of dutasteride up to 40 mg (80 times the therapeutic dose) for 7 days have been administered without significant safety concerns. In a clinical trial, daily doses of 5 mg (10 times the therapeutic dose) were administered to 60 subjects for 6 months with no additional adverse effects to those seen at therapeutic doses of 0.5 mg.
There is no specific antidote for dutasteride. Therefore, in cases of suspected overdosage symptomatic and supportive treatment should be given as appropriate, taking the long half-life of dutasteride into consideration.
Tamsulosin
Should overdosage of tamsulosin lead to hypotension [seeWarnings and Precautions ( ], support of the cardiovascular system is of first importance. Restoration of blood pressure and normalization of heart rate may be accomplished by keeping the patient in the supine position. If this measure is inadequate, then administration of intravenous fluids should be considered. If necessary, vasopressors should then be used and renal function should be monitored and supported as needed. Laboratory data indicate that tamsulosin is 94% to 99% protein bound; therefore, dialysis is unlikely to be of benefit.
6DESCRIPTION
Dutasteride and tamsulosin hydrochloride capsules contain dutasteride (a selective inhibitor of both the type 1 and type 2 isoforms of steroid 5 alpha-reductase, an intracellular enzyme that converts testosterone to DHT and tamsulosin (an antagonist of alpha
- One dutasteride oblong, opaque, yellow gelatin capsule, containing 0.5 mg of dutasteride dissolved in a mixture of butylated hydroxytoluene and mono-and di-glycerides of caprylic/capric acid. The inactive ingredients in the soft-gelatin capsule shell are ferric oxide (yellow), gelatin (from certified BSE-free bovine sources), glycerin, titanium dioxide, lecithin, medium chain triglycerides, propylene glycol, iron oxide black, polyvinyl acetate phthalate, macrogol, and ammonium hydroxide.
- Tamsulosin hydrochloride white to off-white pellets, containing 0.4 mg tamsulosin hydrochloride and the inactive ingredients: methacrylic acid copolymer, sugar sphere, ethylcellulose, polyethylene glycol, triethyl citrate and talc.
The above components are encapsulated in a hard-shell capsule made with the inactive ingredients of hypromellose, FD&C Blue #1, titanium dioxide, shellac, iron oxide black, propylene glycol, FD&C blue #2, FD&C red #40, D&C yellow #10. Blue opaque cap imprinted with “C280” and white opaque body imprinted with “0.5/0.4” in black ink containing white to off white spherical shaped pellets and one oblong, opaque yellow softgel capsule printed with “C300” in black ink.
Dutasteride:Dutasteride is a synthetic 4-azasteroid compound chemically designated as (5α,17β)-N-{2,5 bis(trifluoromethyl)phenyl}-3-oxo-4-azaandrost-1-ene-17-carboxamide. The empirical formula of dutasteride is C 27H 30F 6N 2O 2, representing a molecular weight of 528.5 with the following structural formula:
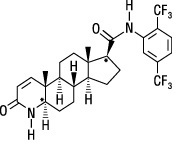
Dutasteride is a white to pale yellow powder with a melting point of 242° to 250°C. It is soluble in ethanol (44 mg/mL), methanol (64 mg/mL), and polyethylene glycol 400 (3 mg/mL), but it is insoluble in water.
Tamsulosin:Tamsulosin hydrochloride is a synthetic compound chemically designated as (-)-( R)-5-[2-[[2-( o-Ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide, monohydrochloride.
The empirical formula of tamsulosin hydrochloride is C
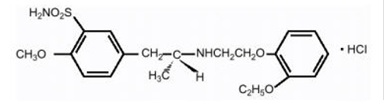
Tamsulosin hydrochloride is a white or almost white crystalline powder that melts with decomposition at approximately 234°C. It is sparingly soluble in water and slightly soluble in methanol, ethanol, acetone, and ethyl acetate.
7CLINICAL STUDIES
The trial supporting the efficacy of dutasteride and tamsulosin hydrochloride capsules was a 4-year multicenter, randomized, double-blind, parallel-group trial (CombAT trial) investigating the efficacy of the coadministration of dutasteride 0.5 mg/day and tamsulosin hydrochloride 0.4 mg/day (n = 1,610) compared with dutasteride alone (n = 1,623) or tamsulosin alone (n = 1,611). Subjects were aged at least 50 years with a serum PSA ≥1.5 ng/mL and <10 ng/mL and BPH diagnosed by medical history and physical examination, including enlarged prostate (≥30 cc) and BPH symptoms that were moderate to severe according to the International Prostate Symptom Score (IPSS). Eighty-eight percent (88%) of the enrolled trial population was white. Approximately 52% of subjects had previous exposure to 5-alpha-reductase inhibitor or alpha-adrenergic antagonist treatment. Of the 4,844 subjects randomly assigned to receive treatment, 69% of subjects in the coadministration group, 67% in the dutasteride group, and 61% in the tamsulosin group completed 4 years of double-blind treatment.
Effect on Symptom Score
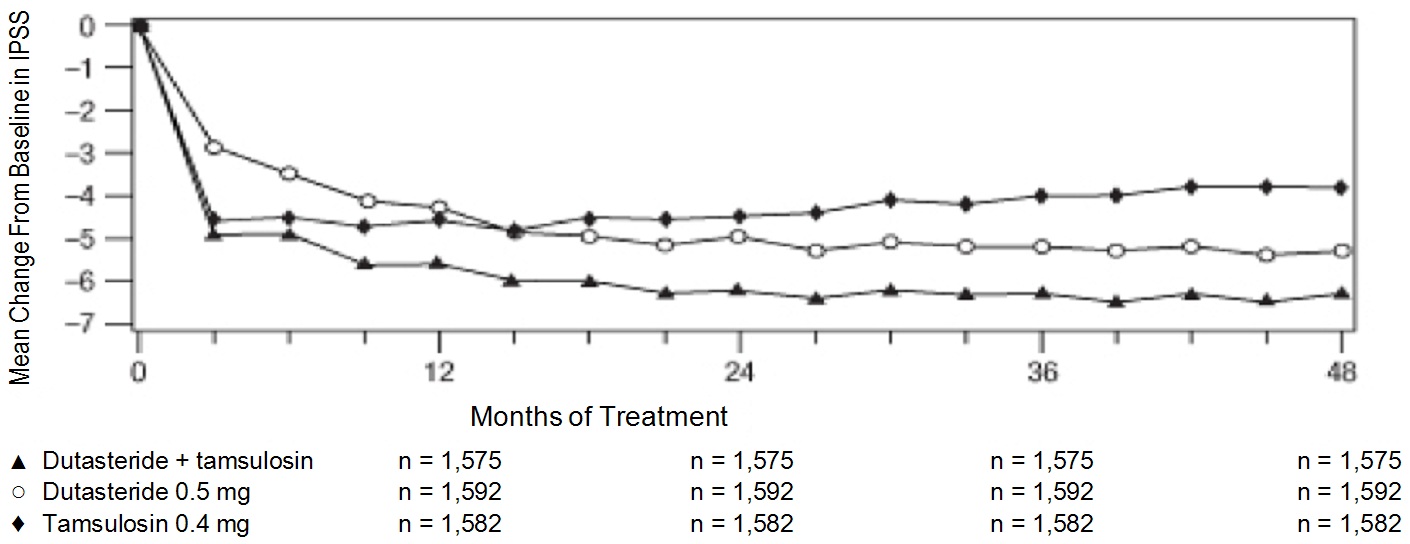
Symptoms were quantified using the first 7 questions of the International Prostate Symptom Score (IPSS). The baseline score was approximately 16.4 units for each treatment group. Coadministration therapy was statistically superior to each of the monotherapy treatments in decreasing symptom score at Month 24, the primary time point for this endpoint. At Month 24, the mean changes from baseline (±SD) in IPSS total symptom scores were -6.2 (±7.14) for the coadministration group, -4.9 (±6.81) for dutasteride, and -4.3 (±7.01) for tamsulosin, with a mean difference between coadministration and dutasteride of -1.3 units ( P<0.001; [95% CI: -1.69, -0.86]), and between coadministration and tamsulosin of -1.8 units ( P<0.001; [95% CI: -2.23, -1.40]). A significant difference was seen by Month 9 and continued through Month 48. At Month 48 the mean changes from baseline (±SD) in IPSS total symptom scores were -6.3 (±7.40) for coadministration, -5.3 (±7.14) for dutasteride, and -3.8 (±7.74) for tamsulosin, with a mean difference between coadministration and dutasteride of -0.96 units ( P<0.001; [95% CI: -1.40, -0.52]), and between coadministration and tamsulosin of -2.5 units ( P<0.001; [95% CI: -2.96, -2.07]). See Figure 1.
Figure 1. International Prostate Symptom Score Change from Baseline over a 48-MonthPeriod (Randomized, Double-blind, Parallel-group Trial [CombAT Trial])
Effect on Acute Urinary Retention (AUR) or the Need for BPH-Related Surgery
After 4 years of treatment, coadministration therapy with dutasteride and tamsulosin did not provide benefit over dutasteride monotherapy in reducing the incidence of AUR or BPH-related surgery.
In separate 2-year randomized, double-blind trials, compared with placebo, dutasteride monotherapy was associated with a statistically significantly lower incidence of AUR (1.8% for dutasteride versus 4.2% for placebo; 57% reduction in risk) and with a statistically significantly lower incidence of BPH-related surgery (2.2% for dutasteride versus. 4.1% for placebo; 48% reduction in risk).
Effect on Maximum Urine Flow Rate
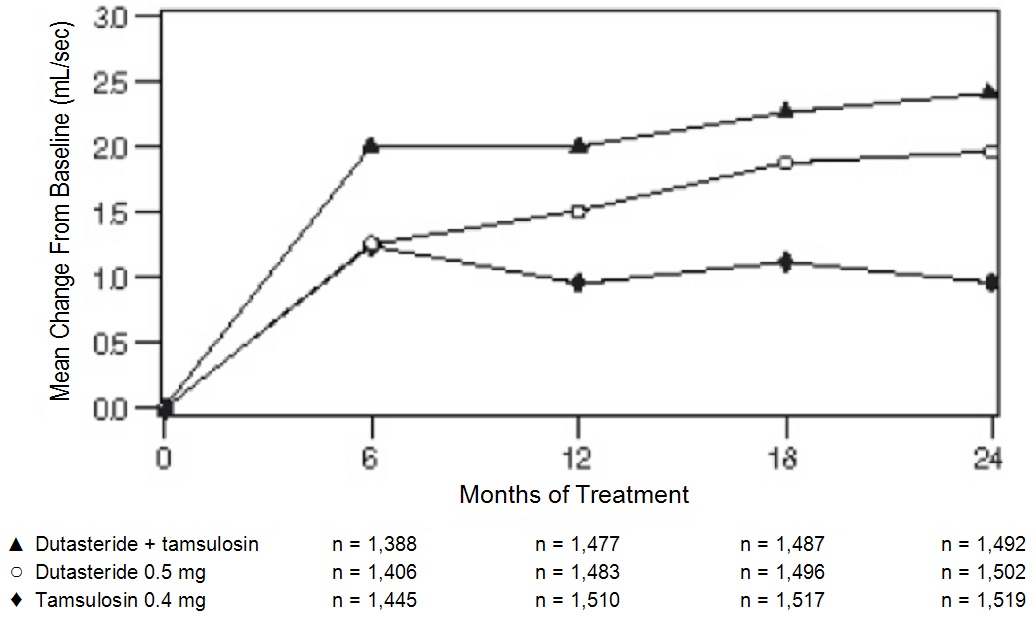
The baseline Q
The additional improvement in Q
Figure 2. Q
Effect on Prostate Volume
The mean prostate volume at trial entry was approximately 55 cc. At Month 24, the primary time point for this endpoint, the mean percent changes from baseline (±SD) in prostate volume were -26.9% (±22.57) for coadministration therapy, -28.0% (±24.88) for dutasteride, and 0% (±31.14) for tamsulosin, with a mean difference between coadministration and dutasteride of 1.1% (
8HOW SUPPLIED/STORAGE AND HANDLING
Dutasteride and tamsulosin hydrochloride capsules, containing 0.5 mg dutasteride and 0.4 mg tamsulosin hydrochloride, are blue, opaque cap imprinted with “C280” and white, opaque body imprinted with “0.5/0.4” in black ink containing white to off-white spherical shaped pellets and one oblong, opaque yellow softgel capsule printed with “C300” in black ink. They are available in bottles with child-resistant closures as follows:
Bottle of 30 (NDC 42291-031-30).
Storeat 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Capsules may become deformed and/or discolored if kept at high temperatures.
Dutasteride is absorbed through the skin. Dutasteride and tamsulosin hydrochloride capsules should not be handled by females who are pregnant or who could become pregnant because of the potential for absorption of dutasteride and the subsequent potential risk to a developing male fetus
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Orthostatic Hypotension
Inform patients about the possible occurrence of symptoms related to orthostatic hypotension, such as dizziness and vertigo, and the potential risk of syncope when taking dutasteride and tamsulosin hydrochloride capsules. Caution patients starting treatment with dutasteride and tamsulosin hydrochloride capsules to avoid situations where injury could result should syncope occur (e.g., driving, operating machinery, performing hazardous tasks). Advise patients to sit or lie down at the first signs of orthostatic hypotension
Drug Interactions
Advise patients that dutasteride and tamsulosin hydrochloride capsules should not be used in combination with strong inhibitors of CYP3A4 [see
PSA Monitoring
Inform patients that dutasteride and tamsulosin hydrochloride capsules reduces serum PSA levels by approximately 50% within 3 to 6 months of therapy, although it may vary for each individual. For patients undergoing PSA screening, increases in PSA levels while on treatment with dutasteride and tamsulosin hydrochloride capsules may signal the presence of prostate cancer and should be evaluated by a healthcare provider
Increased Risk of High-Grade Prostate Cancer
Inform patients that there was an increase in high-grade prostate cancer in men treated with 5-alpha-reductase inhibitors (which are indicated for BPH treatment), including dutasteride, which is a component of dutasteride and tamsulosin hydrochloride capsules, compared with those treated with placebo in trials looking at the use of these drugs to reduce the risk of prostate cancer
Transdermal Exposure of Dutasteride and Tamsulosin hydrochlorideinPregnant or Potentially Pregnant Females -Risk to Male Fetus
Inform patients that dutasteride and tamsulosin hydrochloride capsules should not be handled by females who are pregnant or may potentially be pregnant because of the potential for absorption of dutasteride and the subsequent potential risk to a developing male fetus. Dutasteride can be absorbed through the skin and could result in unintended fetal exposure. If a pregnant or potentially pregnant female comes in contact with leaking dutasteride and tamsulosin hydrochloride capsules, the contact area should be washed immediately with soap and water
Effects on Semen Parameters
Advise men that dutasteride and tamsulosin hydrochloride capsules may affect sperm characteristics but the effect on fertility is unknown
Administration Instructions
Dutasteride and tamsulosin hydrochloride capsules should be swallowed whole and not chewed, crushed, or opened. Dutasteride and tamsulosin hydrochloride capsules may become deformed and/or discolored if kept at high temperatures. If this occurs, capsules should not be used.
Priapism
Inform patients about the possibility of priapism as a result of treatment with dutasteride and tamsulosin hydrochloride capsules or other alpha-adrenergic-antagonist-containing medications. Inform patients that this reaction is extremely rare, but can lead to permanent erectile dysfunction if not brought to immediate medical attention
Blood Donation
Inform men treated with dutasteride and tamsulosin hydrochloride capsules that they should not donate blood until at least 6 months following their last dose to prevent pregnant females from receiving dutasteride through blood transfusion
Intraoperative Floppy Iris Syndrome (IFIS)
Advise patients considering cataract or glaucoma surgery to tell their ophthalmologist that they take or have taken dutasteride and tamsulosin hydrochloride capsules, an alpha adrenergic antagonist-containing product
For Patient Information Leaflet, please visit www.avkare.com.
10PATIENT INFORMATION
Dutasteride (doo tas' ter ide) and Tamsulosin Hydrochloride (tam soo' loe sin hye'' droe klor' ide) Capsules
Dutasteride and tamsulosin hydrochloride capsules are for use by men only.
What are dutasteride and tamsulosin hydrochloride capsules?
Dutasteride and tamsulosin hydrochloride capsules are a prescription medicine that contains 2 medicines: dutasteride and tamsulosin. Dutasteride and tamsulosin hydrochloride capsules are used to treat the symptoms of benign prostatic hyperplasia (BPH) in men with an enlarged prostate. The 2 medications in dutasteride and tamsulosin hydrochloride capsules work in different ways to improve symptoms of BPH. Dutasteride shrinks the enlarged prostate and tamsulosin relaxes muscles in the prostate and neck of the bladder. These 2 medications, when used together, can improve symptoms of BPH better than either medication when used alone.
Do not take dutasteride and tamsulosin hydrochloride capsules if you are:
- pregnant or may be pregnant. Dutasteride and tamsulosin hydrochloride capsules may harm your unborn baby. Pregnant females should not touch dutasteride and tamsulosin hydrochloride capsules. If a female who is pregnant with a male baby gets enough dutasteride and tamsulosin hydrochloride capsules in her body by swallowing or touching dutasteride and tamsulosin hydrochloride capsules, the male baby may be born with sex organs that are not normal. If a pregnant female comes in contact with leaking dutasteride and tamsulosin hydrochloride capsules, the contact area should be washed immediately with soap and water.
- allergic to dutasteride, tamsulosin, or any of the ingredients in dutasteride and tamsulosin hydrochloride capsules. See the end of this leaflet for a complete list of ingredients in dutasteride and tamsulosin hydrochloride capsules.
- taking another medicine that contains an alpha-blocker.
- allergic to other 5-alpha-reductase inhibitors, for example, PROSCAR (finasteride) tablets.
Before you take dutasteride and tamsulosin hydrochloride capsules, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of low blood pressure
- take medicines to treat high blood pressure
- plan to have cataract or glaucoma surgery
- have liver problems
- are allergic to sulfa medications
- have any other medical conditions
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Dutasteride and tamsulosin hydrochloride capsules and other medicines may affect each other, causing side effects. Dutasteride and tamsulosin hydrochloride capsules may affect the way other medicines work, and other medicines may affect how dutasteride and tamsulosin hydrochloride capsules works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take dutasteride and tamsulosin hydrochloride capsules?
- Take dutasteride and tamsulosin hydrochloride capsules exactly as your healthcare provider tells you to take it.
- Swallow dutasteride and tamsulosin hydrochloride capsules whole. Do not crush, chew, or open dutasteride and tamsulosin hydrochloride capsules because the contents of the capsule may irritate your lips, mouth, or throat.
- Take your dutasteride and tamsulosin hydrochloride capsules 1 time each day, about 30 minutes after the same meal every day. For example, you may take dutasteride and tamsulosin hydrochloride capsules 30 minutes after dinner every day.
- If you miss a dose, you can take it later that same day, 30 minutes after a meal. Do not take 2 dutasteride and tamsulosin hydrochloride capsules in the same day. If you stop or forget to take dutasteride and tamsulosin hydrochloride capsules for several days, talk with your healthcare provider before starting again.
- If you take too much dutasteride and tamsulosin hydrochloride capsules, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking dutasteride and tamsulosin hydrochloride capsules?
- Avoid driving, operating machinery, or other dangerous activities when starting treatment with dutasteride and tamsulosin hydrochloride capsules until you know how dutasteride and tamsulosin hydrochloride capsules affects you. Dutasteride and tamsulosin hydrochloride capsules can cause a sudden drop in your blood pressure, especially at the start of treatment. A sudden drop in blood pressure may cause you to faint, feel dizzy or lightheaded.
- You should not donate blood while taking dutasteride and tamsulosin hydrochloride capsules or for 6 months after you have stopped dutasteride and tamsulosin hydrochloride capsules. This is important to prevent pregnant females from receiving dutasteride and tamsulosin hydrochloride capsules through blood transfusions.
What are the possible side effects of dutasteride and tamsulosin hydrochloride capsules?
Dutasteride and tamsulosin hydrochloride capsulesmay cause serious side effects including:
- Decreased blood pressure.Dutasteride and tamsulosin hydrochloride capsules may cause a sudden drop in your blood pressure upon standing from a sitting or lying position, especially at the start of treatment. Symptoms of low blood pressure may include:
- fainting
- dizziness
- feeling lightheaded
- Rare and serious allergic reactions, including:
- swelling of your face, tongue, or throat
- difficulty breathing
- serious skin reactions, such as skin peeling
Get medical help right away if you have these serious allergic reactions.
- Higher chance of a more serious form of prostate cancer.
- Eye problems during cataract or glaucoma surgery.During cataract or glaucoma surgery, a condition called Intraoperative Floppy Iris Syndrome (IFIS) can happen if you take or have taken dutasteride and tamsulosin hydrochloride capsules in the past. If you need to have cataract or glaucoma surgery, tell your surgeon if you take or have taken dutasteride and tamsulosin hydrochloride capsules.
- A painful erection that will not go away.Rarely, dutasteride and tamsulosin hydrochloride capsules can cause a painful erection (priapism), which cannot be relieved by having sex. If this happens, get medical help right away. If priapism is not treated, there could be lasting damage to your penis, including not being able to have an erection.
The most common side effects of dutasteride and tamsulosin hydrochloride capsules include:
- ejaculation problems*
- trouble getting or keeping an erection (impotence)*
- a decrease in sex drive (libido)*
- dizziness
- enlarged or painful breasts. If you notice breast lumps or nipple discharge, you should talk to your healthcare provider.
- runny nose
*Some of these events may continue after you stop taking dutasteride and tamsulosin hydrochloride capsules.
Depressed mood has been reported in patients receiving dutasteride, an ingredient of dutasteride and tamsulosin hydrochloride capsules.
Dutasteride, an ingredient of dutasteride and tamsulosin hydrochloride capsules, has been shown to reduce sperm count, semen volume, and sperm movement. However, the effect of dutasteride and tamsulosin hydrochloride capsules on male fertility is not known.
Prostate-Specific Antigen (PSA) Test:Your healthcare provider may check you for other prostate problems, including prostate cancer before you start and while you take dutasteride and tamsulosin hydrochloride capsules. A blood test called PSA (prostate-specific antigen) is sometimes used to see if you might have prostate cancer. Dutasteride and tamsulosin hydrochloride capsules will reduce the amount of PSA measured in your blood. Your healthcare provider is aware of this effect and can still use PSA to see if you might have prostate cancer. Increases in your PSA levels while on treatment with dutasteride and tamsulosin hydrochloride capsules (even if the PSA levels are in the normal range) should be evaluated by your healthcare provider. These are not all the possible side effects with dutasteride and tamsulosin hydrochloride capsules. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store dutasteride and tamsulosin hydrochloride capsules?
- Store dutasteride and tamsulosin hydrochloride capsules at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
- Dutasteride and tamsulosin hydrochloride capsules may become deformed and/or discolored if kept at high temperatures.
- Do not use or touch dutasteride and tamsulosin hydrochloride capsules if your capsules are deformed, discolored, or leaking.
- Safely throw away medicine that is no longer needed.
Keep dutasteride and tamsulosin hydrochloride capsules and all medicines out of the reach of children.
General information about the safe and effective use of dutasteride and tamsulosin hydrochloride capsules.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use dutasteride and tamsulosin hydrochloride capsules for a condition for which it was not prescribed. Do not give dutasteride and tamsulosin hydrochloride capsules to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about dutasteride and tamsulosin hydrochloride capsules that is written for health professionals.
For more information, call 1-855-361-3993.
What are the ingredients in dutasteride and tamsulosin hydrochloride capsules?
Active ingredients:dutasteride and tamsulosin hydrochloride
Inactive ingredients:butylated hydroxytoluene, ethylcellulose, gelatin, glycerin, lecithin, medium chain triglycerides, methacrylic acid copolymer, mono- and di-glycerides of capryl/capric acid, polyethylene glycol, sugar spheres, talc, triethyl citrate, iron oxide yellow, hypromellose, titanium dioxide, D&C yellow #10, iron oxide black, FD&C blue #2, FD&C blue #1, propylene glycol, FD&C red #40, shellac, polyvinyl acetate phthalate, macrogol, ammonium hydroxide.
Trademarks are the properties of their respective owners.
This Patient Information has been approved by the U.S. Food and Drug Administration.
For Patient Information Leaflet, please visit please visit www.avkare.com
Manufactured for:
AvKARE
Pulaski, TN 38478
Mfg. Rev. 10/21
AV 11/23 (M)
11PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
