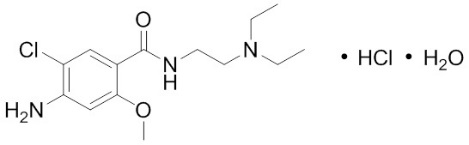
Metoclopramide
What is Reglan (Metoclopramide)?
Nausea, vomiting and slow digestion can disrupt everyday life, making even simple activities feel exhausting. Metoclopramide is a trusted medication that helps relieve these symptoms by improving how the stomach and intestines move food. It’s widely prescribed for people dealing with delayed stomach emptying (gastroparesis), reflux-related symptoms, or nausea caused by illness, surgery, or certain treatments.
Metoclopramide belongs to a class of drugs called prokinetic agents, which enhance the movement of the gastrointestinal (GI) tract. It has been in medical use for decades and remains a key treatment option when other remedies haven’t provided enough relief.
What does Metoclopramide do?
Metoclopramide is primarily used to treat nausea and vomiting from various causes including migraine, cancer therapy or after surgery. It is also effective for managing diabetic gastroparesis, a condition where delayed stomach emptying leads to bloating, heartburn and a feeling of fullness after small meals.
By speeding up stomach emptying and reducing reflux, Metoclopramide helps patients feel more comfortable and able to eat normally again. Many people experience noticeable relief within days of starting treatment, especially when taken under medical supervision.
According to studies, Metoclopramide can significantly reduce nausea intensity and improve meal tolerance in individuals with diabetic gastroparesis, making it a common first-line choice for this condition (FDA, 2023).
How does Metoclopramide work?
Metoclopramide works by targeting specific chemical messengers in the brain and digestive system. It blocks dopamine receptors in the brain’s chemoreceptor trigger zone, the area that controls nausea and vomiting, preventing signals that cause these symptoms.
At the same time, it enhances the natural movements of the stomach and intestines by increasing the action of acetylcholine, a neurotransmitter that stimulates GI muscle contractions. This dual action helps food move more efficiently through the stomach and intestines, reducing discomfort from bloating or delayed digestion.
Clinically, this mechanism matters because it not only helps relieve nausea but also addresses the underlying issue, impaired motility, making Metoclopramide particularly effective for chronic digestive conditions like gastroparesis.
Metoclopramide side effects
Like all medications, Metoclopramide can cause side effects, though not everyone experiences them. Most are mild and improve with continued use or dose adjustment.
Common side effects may include:
- Drowsiness or fatigue
- Restlessness or mild anxiety
- Diarrhea
- Headache
Less common but more serious side effects include:
- Involuntary muscle movements (tardive dyskinesia), especially with long-term use
- Muscle stiffness, tremors or difficulty moving
- Depression or mood changes
Because of the risk of tardive dyskinesia, Metoclopramide is usually prescribed for short-term use, typically not exceeding 12 weeks (Mayo Clinic, 2023).
Who should avoid Metoclopramide? This medication is generally not recommended for individuals with Parkinson’s disease, bowel obstruction, bleeding or a history of movement disorders. Always discuss your full medical history with your doctor before starting the drug.
When to seek medical help: Seek immediate attention if you develop uncontrollable facial movements, confusion, or signs of an allergic reaction such as rash, swelling or difficulty breathing.
Metoclopramide dosage
Metoclopramide is available in several forms, including tablets, oral solutions and injections. Your doctor will determine the best form and schedule based on your condition and response to treatment.
Because the drug acts on both the digestive system and the brain, your healthcare provider may monitor your neurological health and overall well-being during therapy. For patients on long-term treatment, periodic evaluations help detect early signs of movement-related side effects.
Older adults or those with liver or kidney disease may require adjusted dosing, as their bodies process medications more slowly. It’s also important not to exceed the prescribed duration of therapy, since long-term use raises the risk of side effects.
Does Metoclopramide have a generic version?
Yes, Metoclopramide is available as a generic medication that is FDA-approved and equally effective as brand-name products such as Reglan. Generic versions contain the same active ingredient, strength, and safety profile, offering a more affordable option for patients.
Generic Metoclopramide is available in multiple forms, including oral tablets and injectable preparations, making it accessible for both outpatient and hospital use.
Conclusion
Metoclopramide plays a vital role in managing nausea, vomiting and disorders related to slow stomach emptying. By enhancing GI movement and calming the brain’s nausea centers, it provides relief and helps patients regain normal eating patterns and daily comfort.
While generally safe and effective, it requires careful use, particularly with long-term therapy to prevent side effects. Regular communication with your healthcare provider ensures that treatment remains safe, tailored, and beneficial for your individual needs.
Metoclopramide can make a significant difference for those struggling with chronic digestive discomfort or persistent nausea when used responsibly under medical guidance. If prescribed, take it exactly as directed and report any unusual symptoms promptly, your care team is there to help you achieve the best results safely.
References
- U.S. Food and Drug Administration (FDA). (2023). Metoclopramide Medication Guide. https://www.fda.gov/
- Mayo Clinic. (2023). Metoclopramide (Oral Route) Description and Precautions. https://www.mayoclinic.org/
- MedlinePlus. (2023). Metoclopramide: Drug Information. https://medlineplus.gov/
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- Reglan can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. There is no known treatment for TD. The risk of developing TD increases with duration of treatment and total cumulative dosage
- Discontinue Reglan in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after Reglan is stopped
- Avoid treatment with Reglan for longer than 12 weeks because of the increased risk of developing TD with longer-term use 2.2, 2.3)].
- Treatment for 4 to 12 weeks of symptomatic, documented gastroesophageal reflux in adults who fail to respond to conventional therapy.
- Relief of symptoms in adults with acute and recurrent diabetic gastroparesis.
- 5 mg metoclopramide: green, elliptical-shaped, debossed “REGLAN” over “5” on one side and “ANI” on the opposite side
- 10 mg metoclopramide: white, double edge scored, capsule-shaped, debossed “REGLAN” on one side and “ANI 10” on the opposite side
- In patients with a history of tardive dyskinesia (TD) or a dystonic reaction to metoclopramide
- When stimulation of gastrointestinal motility might be dangerous (e.g., in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation).
- In patients with pheochromocytoma or other catecholamine-releasing paragangliomas. Reglan may cause a hypertensive/pheochromocytoma crisis, probably due to release of catecholamines from the tumor
- In patients with epilepsy. Reglan may increase the frequency and severity of seizures
- In patients with hypersensitivity to metoclopramide. Reactions have included laryngeal and glossal angioedema and bronchospasm
- Tardive dyskinesia
- Other extrapyramidal effects
- Neuroleptic malignant syndrome
- Depression
- Hypertension
- Fluid retention
- Hyperprolactinemia
- Effects on the ability to drive and operate machinery
- Tardive dyskinesia, acute dystonic reactions, drug-induced parkinsonism, akathisia, and other extrapyramidal symptoms
- Convulsive seizures
- Hallucinations
- Restlessness, drowsiness, fatigue, and lassitude occurred in approximately 10% of patients who received 10 mg four times daily. Insomnia, headache, confusion, dizziness, or depression with suicidal ideation occurred less frequently.
- Neuroleptic malignant syndrome, serotonin syndrome (in combination with serotonergic agents).
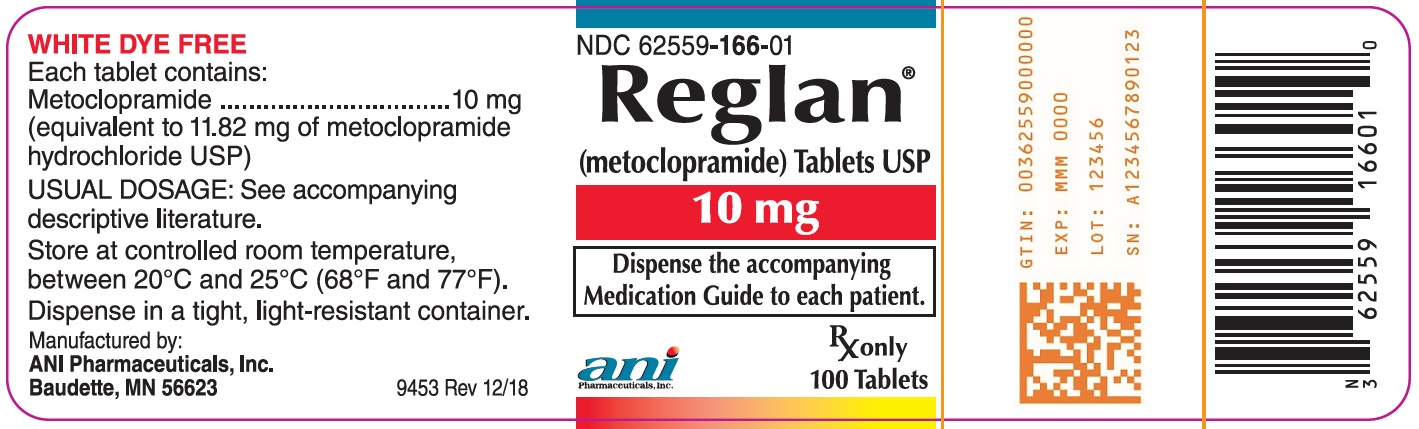
- Each Reglan 5 mg tablet contains 5 mg metoclopramide (equivalent to 5.91 mg of metoclopramide hydrochloride USP). Inactive ingredients consist of corn starch, D&C Yellow 10 Aluminum Lake, FD&C Blue 1 Aluminum Lake, lactose, microcrystalline cellulose, silicon dioxide, and stearic acid.
- Each Reglan 10 mg tablet contains 10 mg metoclopramide (equivalent to 11.82 mg metoclopramide hydrochloride USP). Inactive ingredients consist of magnesium stearate, mannitol, microcrystalline cellulose, and stearic acid.
- Tardive dyskinesia and other extrapyramidal reactions
- Neuroleptic malignant syndrome
- Depression and/or possible suicidal ideation