Somavert
What is Somavert (Pegvisomant)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Background: Lipodystrophy (LD) syndromes are a group of rare disorders that affect how a person s body can store and use fat tissue. Many people with LDs become severely insulin resistant. Some people are insulin resistant because of a variant in the insulin receptor gene. Insulin resistance causes many health problems.
Summary: Acromegaly, a chronic condition characterized by growth hormone (GH) and, in turn, insulin-like growth factor-1 (IGF-I) excess, is burdened by a series of systemic and metabolic comorbidities that strongly impair quality of life (QoL) and life expectancy. Amongst them, a specific acromegalic osteopathy has been discovered, characterized by fragility fractures associated with high bone turnover, wh...
Summary: Non-interventional observational study, to identify safety and effectiveness of Somavert during the post-marketing period based on the Korean RMP as required by the regulations of Ministry of Food and Drug Safety (MFDS)
Related Latest Advances
Brand Information
- Hypoglycemia Associated with GH Lowering in Patients with Diabetes Mellitus
- Liver Toxicity
- Cross-Reactivity with GH Assays
- Lipohypertrophy
- Systemic Hypersensitivity
- Not to use SOMAVERT if they are allergic to SOMAVERT or anything in it.
- They will need blood testing to check IGF-1 levels and liver tests before and during treatment with SOMAVERT and that the dose of SOMAVERT may be changed based on the results of these tests.
- SOMAVERT has not been studied in pregnant women and instruct them to notify their healthcare provider as soon as they are aware that they are pregnant.
- It is not known whether SOMAVERT is excreted in human milk and instruct them to notify their healthcare provider if they plan to do so.
- Pregnancy: Inform female patients that treatment with SOMAVERT may result in unintended pregnancy
- The most common reported adverse reactions are injection site reaction, elevations of liver tests, pain, nausea, and diarrhea.
- If they have liver test elevations they may need to have more frequent liver tests and/or discontinue SOMAVERT. Instruct patients to immediately discontinue therapy and contact their physician if they become jaundiced.
- GH-secreting tumors may enlarge in people with acromegaly and that these tumors need to be watched carefully and monitored by MRI imaging.
- Thickening under the skin may occur at the injection site that could lead to lumps and that switching sites may prevent or lessen this.
- If they have diabetes mellitus, they may require careful monitoring and dose reductions of insulin and/or oral hypoglycemic agents while on SOMAVERT.
- If they take opioids, they may need higher SOMAVERT doses to achieve appropriate IGF-1 suppression.

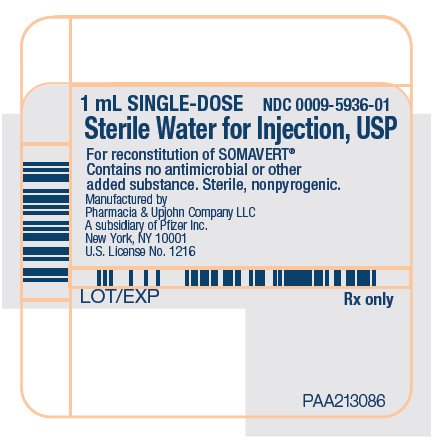
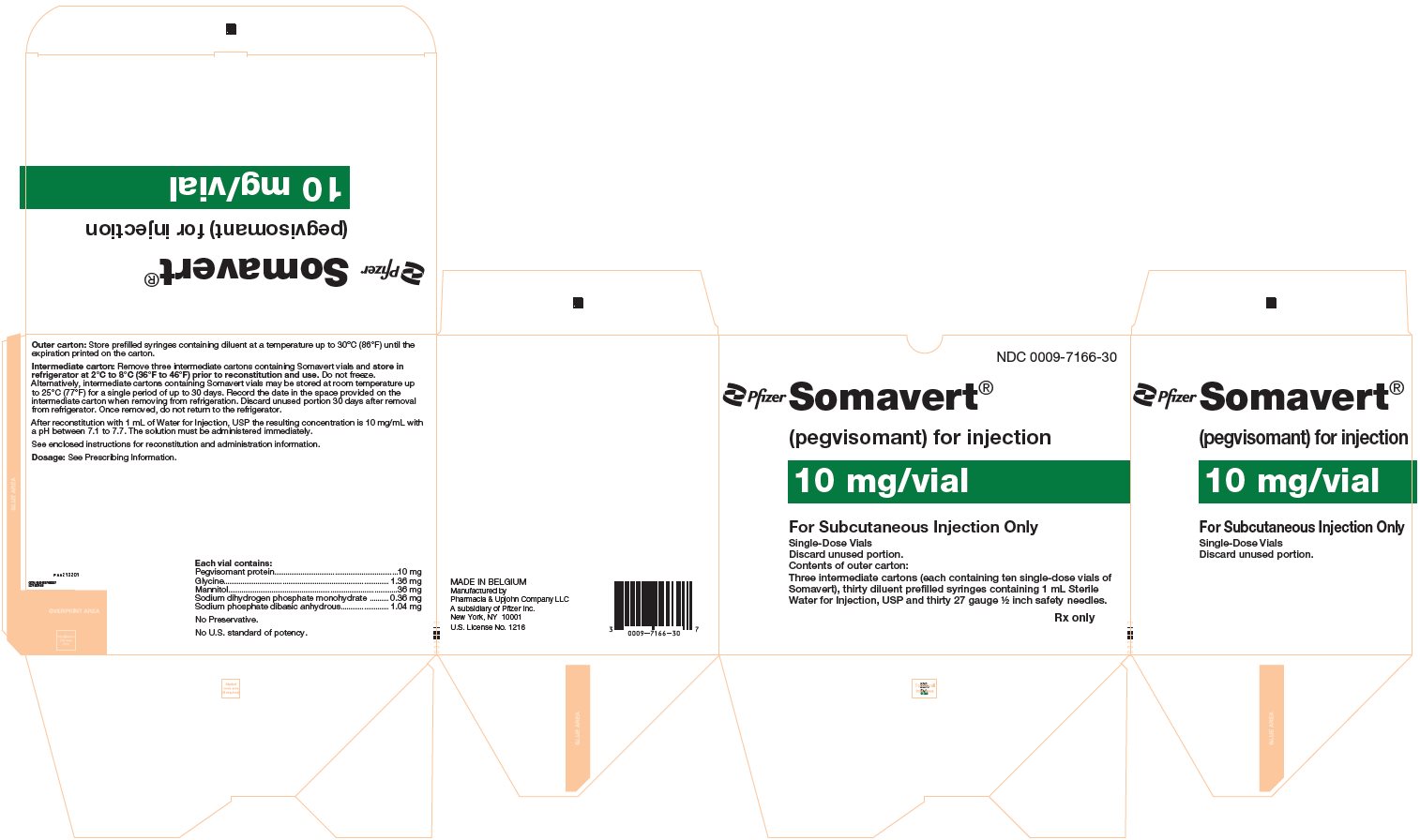
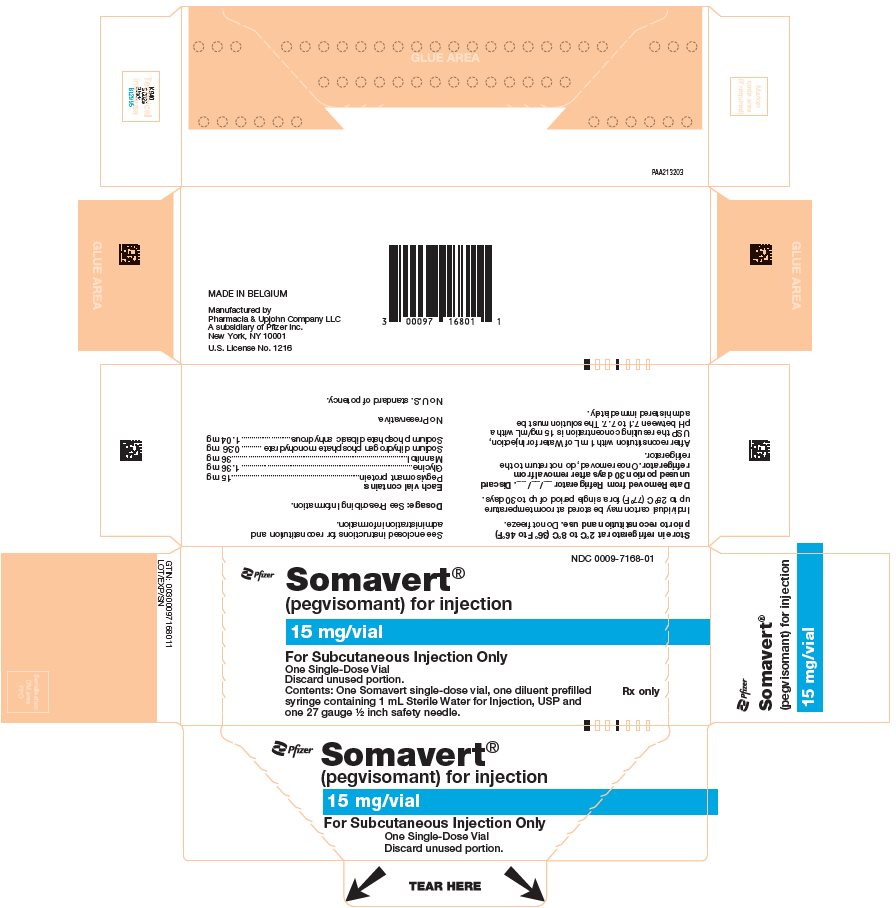
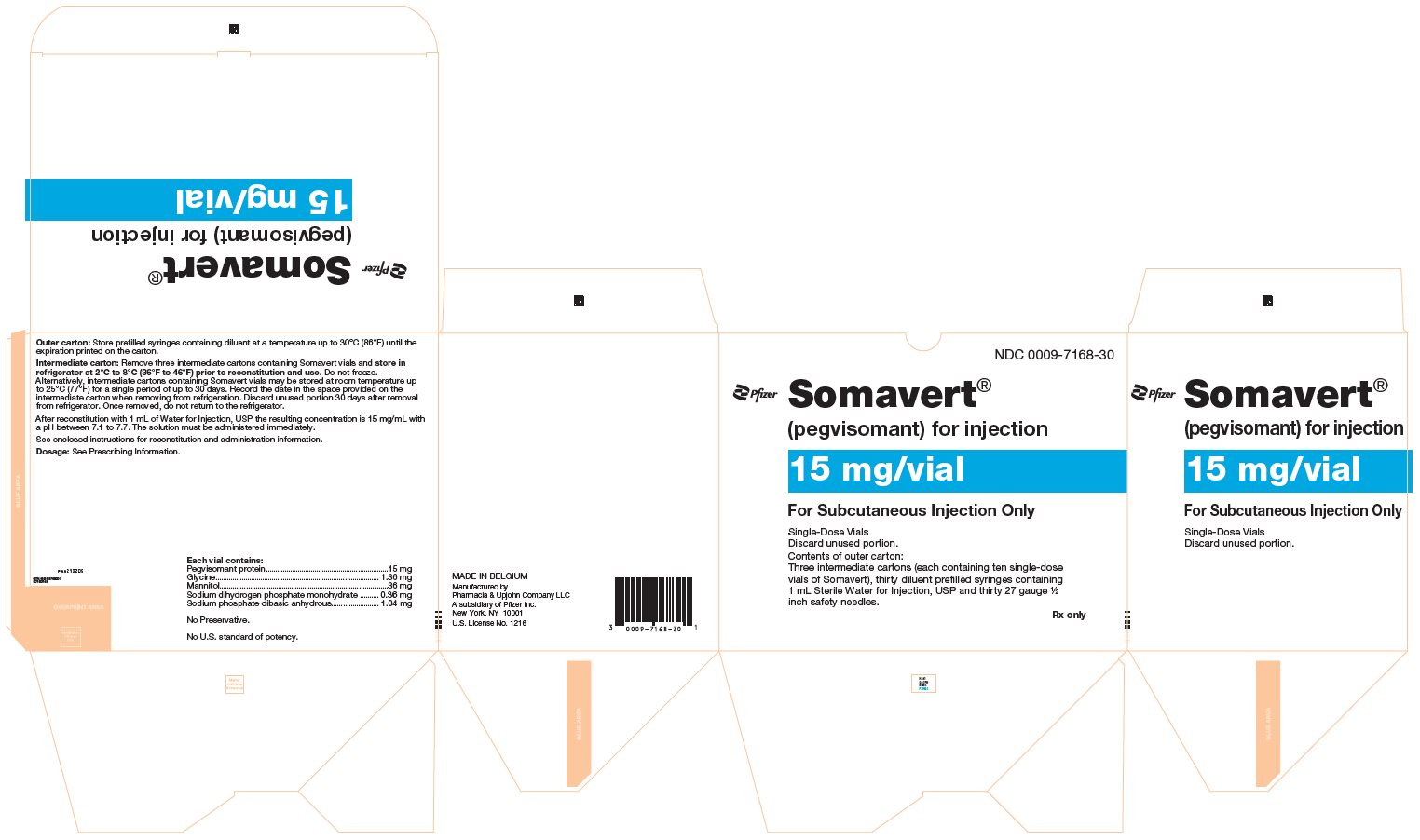
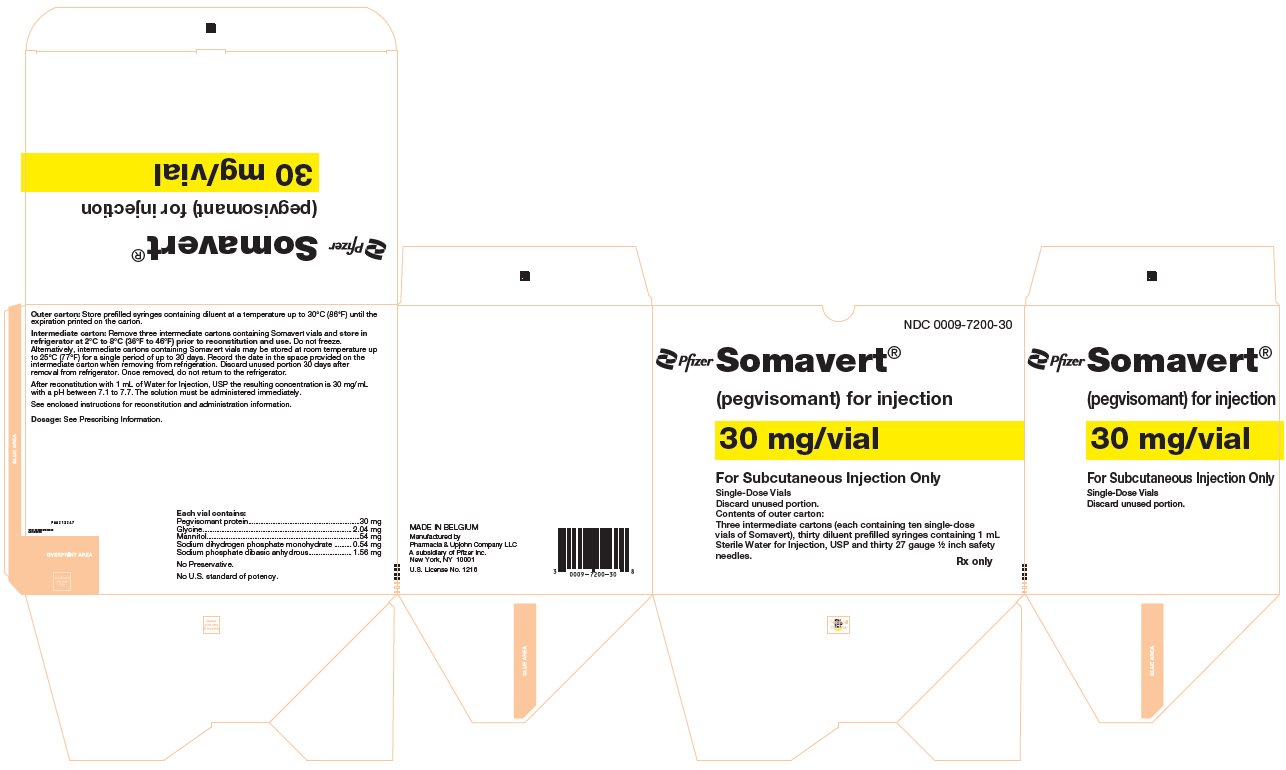
(pegvisomant)
for injection, for subcutaneous use
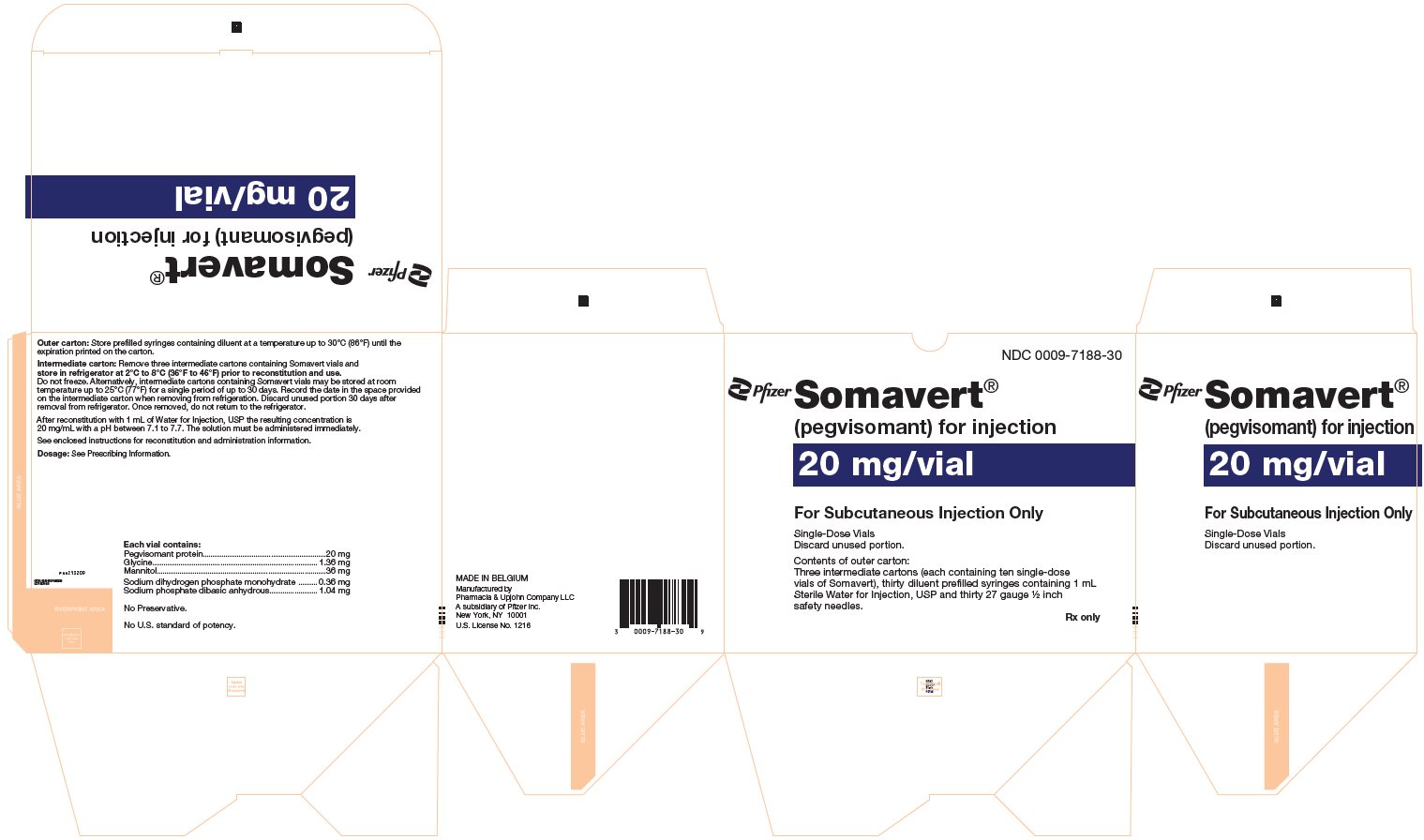
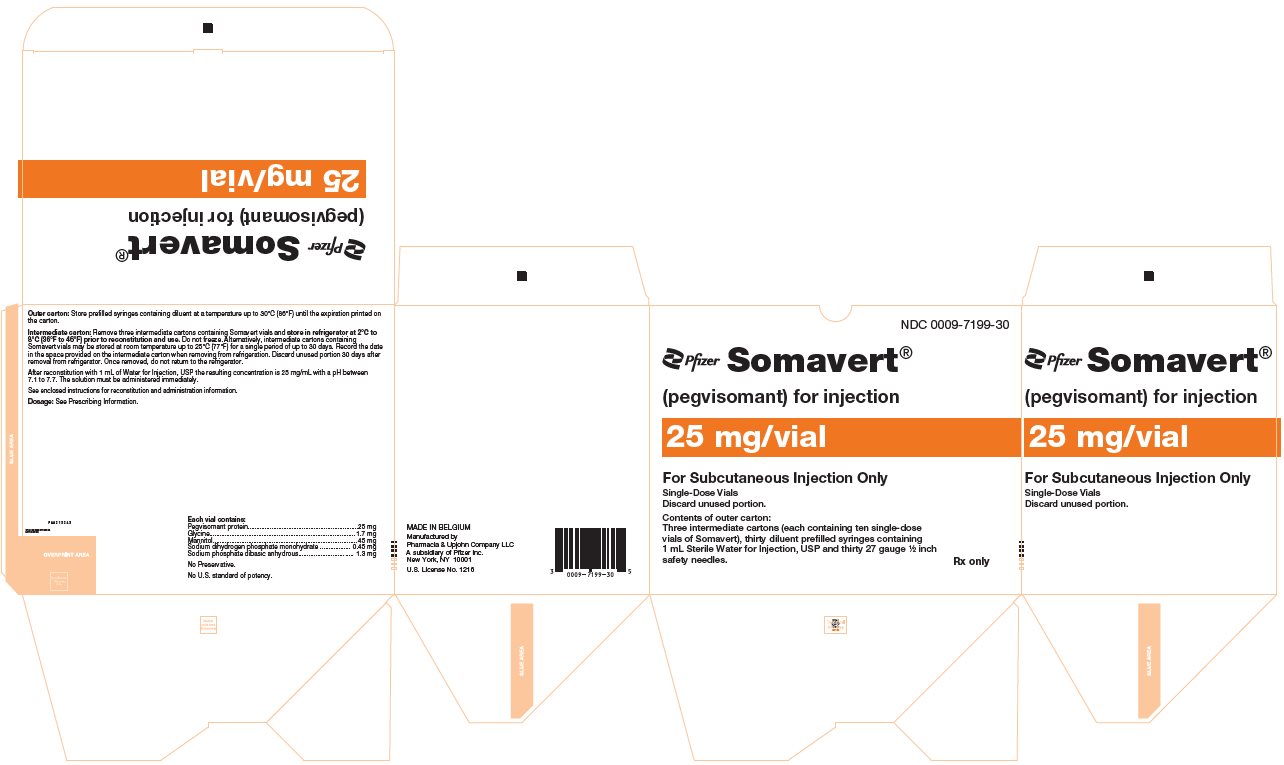
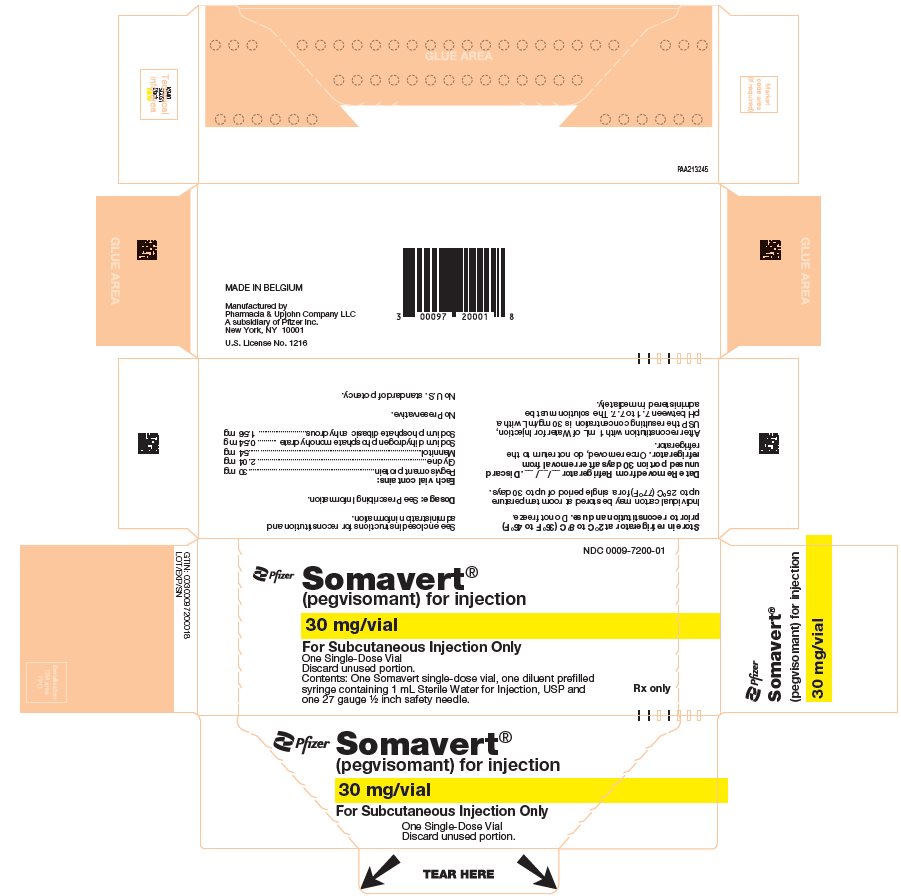
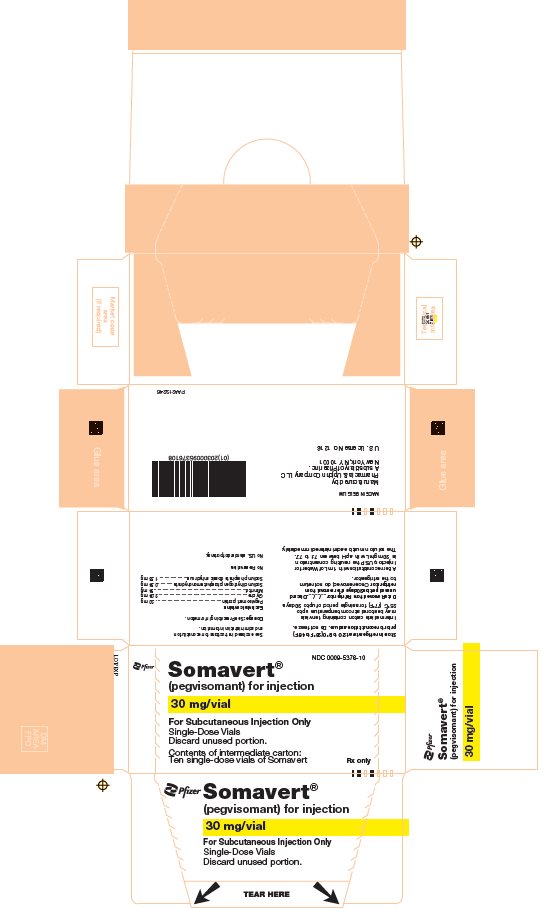
- One Day Package (containing one single-dose vial of SOMAVERT powder, a prefilled syringe, and a safety needle)
- 30-Day Package (containing three intermediate cartons of 10 single-dose vials of SOMAVERT powder, 30 prefilled syringes, and 30 safety needles)
- Before you mix the SOMAVERT powder and the liquid:
- Throw away the SOMAVERT vials after the expiration date printed on the carton or the discard date, whichever is sooner.
- The prefilled syringes may be stored at a temperature up to 86°F (30°C) until the expiration date printed on the carton. After that time, they should be thrown away.
- Do not freeze SOMAVERT.
- Do not share your SOMAVERT syringes or needles with other people. You may give other people a serious infection, or get an infection from them.
- SOMAVERT comes in a vial as a white block of powder. You must mix SOMAVERT with a liquid (diluent) before you can use it. The liquid comes in a single-dose prefilled syringe labeled 'Sterile Water for Injection'.
- You must use the mixed SOMAVERT immediately after you mix it. If you have not used the mixed SOMAVERT immediately, throw it away.
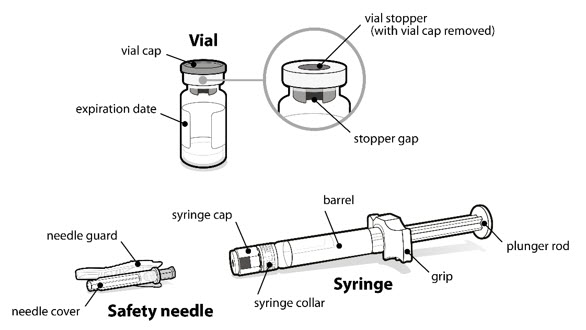
- A vial of SOMAVERT powder.
- A prefilled syringe.
- A safety needle.
- A cotton ball.
- An alcohol swab.
- A sharps disposal container.
- Only mix SOMAVERT and the liquid when you are ready to inject your dose.
- SOMAVERT One Day Package: If refrigerated, remove the package and allow it to come to room temperature in a safe place for at least
- SOMAVERT 30-Day Package: If refrigerated, remove a single vial from the intermediate carton and allow it to come to room temperature in a safe place for at least
- Do not heat the vial or syringe by using a heat source such as hot water or microwave. Let it warm up on its own.
- Wash your hands with soap and water, and dry completely.
- Peel open the packaging of the syringe and safety needle to make it easier to pick up each item as you prepare for your injection.
- Do not use the syringe or vial if:
- they are damaged or faulty
- the expiration date has passed
- it has been frozen, even if it has now thawed (syringe only)
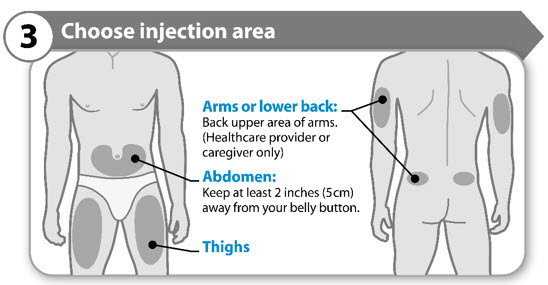
- Choose a different location within an area for each injection.
- Avoid bony areas or areas that are bruised, red, sore or hard, or areas that have scars or skin conditions.
- Clean the injection area with the alcohol swab as instructed by your healthcare provider.
- Allow the injection area to dry.
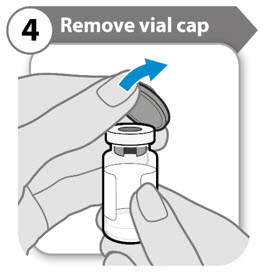
- Remove the cap from the vial.
- Throw the cap away. It is not needed again.
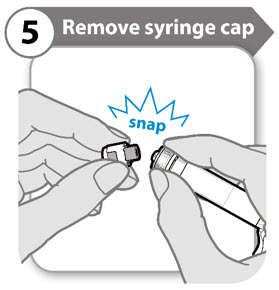
- Snap off the syringe cap leaving the syringe collar in place. It may take more effort to snap off than you might expect.
- Throw the syringe cap away. It is not needed again.
- Keep the syringe upright to avoid leakage.
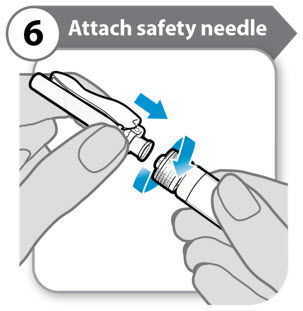
- Push down and twist the safety needle firmly onto the syringe as far as it will go.
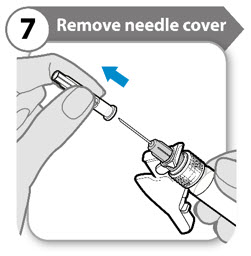
- Fold the needle guard out of the way of the needle cover.
- Carefully pull the needle cover straight off.
- Throw the needle cover away. It is not needed again.
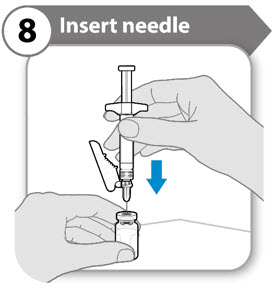
- Push the needle through the center of vial stopper, as shown.
- Support the syringe while the needle is in the vial stopper to prevent bending the needle.
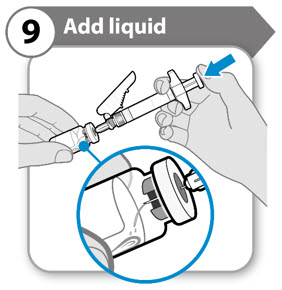
- Tilt both the vial and syringe at an angle, as shown.
- Push the plunger rod down
- Caution: Do not squirt the liquid directly onto the powder. This creates foam. Foam makes the medicine unusable.
- Do not withdraw the needle yet.
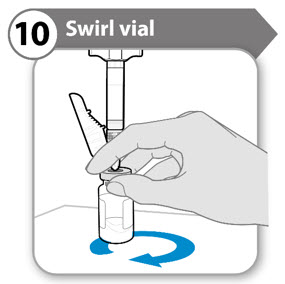
- Support both the syringe and vial in 1 hand, as shown.
- Gently and
- Continue swirling the liquid until all the powder has fully dissolved.
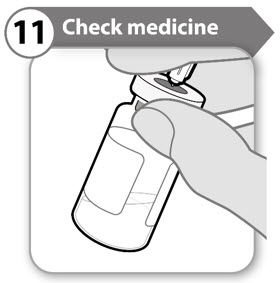
- Keeping the needle in the vial, look carefully at the medicine. It must be clear and free of particles.
- Do not use if:
- the medicine is cloudy or hazy
- the medicine has any color at all
- there are any particles or foam in the vial
- If you have any doubts about your medication go to
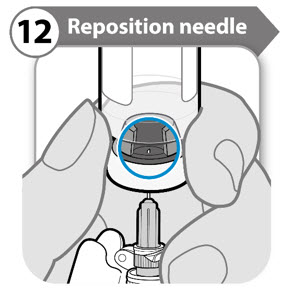
- Turn the vial so that you can see the stopper gap, as shown.
- Pull the needle down so that the needle tip is at the lowest point in the liquid. This will help you to draw off as much liquid as possible.
- Check that the plunger rod has not moved. If the plunger rod has moved, then push it back all the way into the syringe. This ensures that all air is removed from the syringe before you draw off the dose.
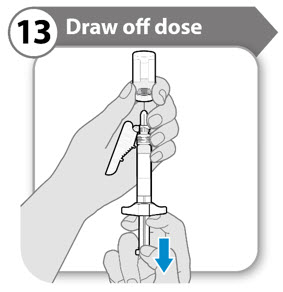
- Slowly pull back the plunger rod to withdraw as much medicine as possible from the vial.
- Pull the needle out of the vial.
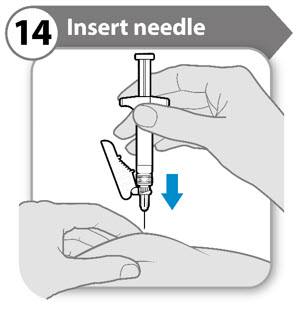
- Gently pinch the skin at the site of injection.
- Insert the needle to its full depth into the pinched skin.
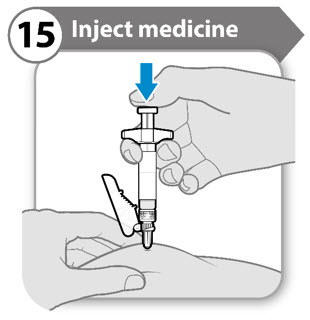
- Push the plunger rod down slowly until the barrel is empty.
- Release the pinched skin and pull the needle straight out.
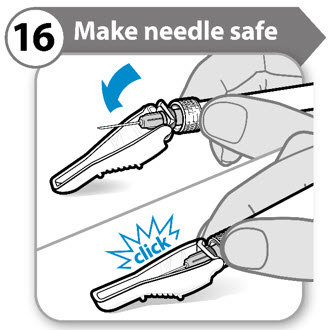
- Fold the needle guard over the needle.
- Note: You will hear a click when the needle guard has been locked.
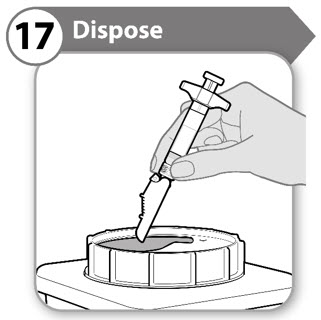
- Put your used syringes in a FDA cleared sharps disposal container right away after use.
- Do not throw away (dispose of) syringes in your household trash.
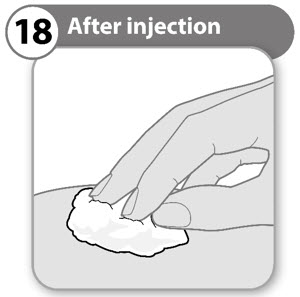
- If necessary, use a clean cotton ball and press lightly on the injection area.
- Do not rub the area.
- Clean the vial stopper with a fresh alcohol wipe, and leave it to dry completely. If you are unable to clean the stopper, do not use the vial.
- Do not use it even if it looks undamaged. Dispose of the syringe in the same way as a used syringe. You will need a replacement syringe.
- Only 1 time. Withdrawing and reinserting greatly increases the risk of needle damage, and will blunt the needle. This can cause discomfort and increases risk of skin damage and infection. There is also a risk you may lose some of the medicine.
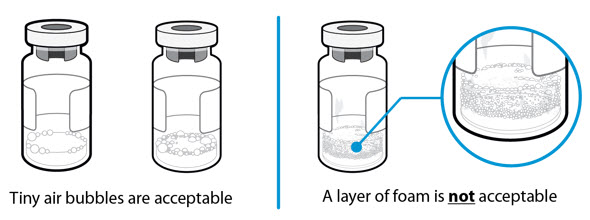
- No. Never shake the vial. Shaking can destroy the medicine and create foam. The powder may take a few minutes to dissolve fully, so continue swirling the vial gently until the liquid is completely clear.
- Foam looks like a mass of small bubbles that float as a layer to the top of the liquid. Do not inject SOMAVERT if it has foamed.
- Press the plunger very slowly so that the liquid gently runs down the inside of the vial. Do not spray the liquid directly onto the powder, because this creates foam. This will also reduce the swirling time and allow more of the medicine to be drawn off.
- Tiny air bubbles in the liquid are normal and are safe to inject. However, it is possible to accidently draw air into the syringe, which should be removed before injecting. Bubbles or air gaps that float to the top of the liquid should be pushed back out into the vial.
- The shape of the vial means that a very small amount of the medicine will be left behind in the vial. This is normal. To ensure that only a trace of medicine remains, make sure the needle tip is as low as it can be in the vial when drawing off your dose.
- For more information, go to
- made of heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use, leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.