Brand Name
Levobunolol
View Brand InformationFDA approval date: March 04, 1994
Classification: beta-Adrenergic Blocker
Form: Solution
What is Levobunolol?
Levobunolol hydrochloride ophthalmic solution has been shown to be effective in lowering intraocular pressure and may be used in patients with chronic open-angle glaucoma or ocular hypertension.
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Levobunolol Hydrochloride (Levobunolol Hydrochloride)
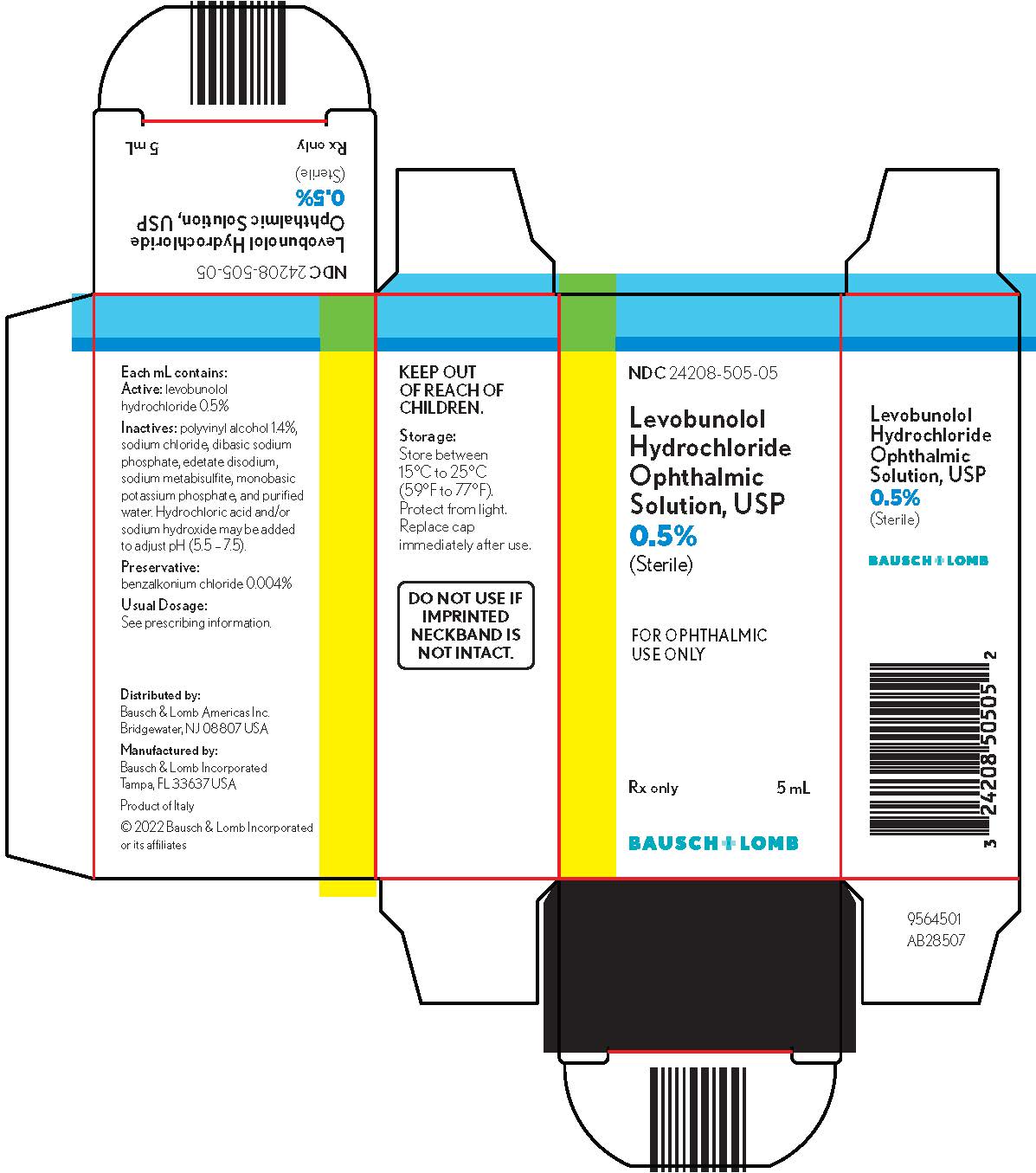
1DESCRIPTION
Levobunolol hydrochloride ophthalmic solution USP, 0.5% is a noncardioselective beta-adrenoceptor blocking agent for ophthalmic use.
Levobunolol hydrochloride is represented by the following structural formula:
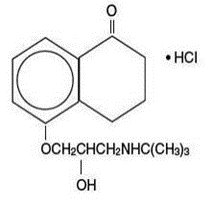
Mol. Formula C
Mol. Wt. 327.85
Chemical Name:(–)-5-[3-( tert-Butylamino)-2-hydroxypropoxy]-3,4-dihydro-1(2 H)- naphthalenone hydrochloride.
Each mL of 0.5% contains:
Active:levobunolol hydrochloride 0.5%; Inactives:polyvinyl alcohol 1.4%, sodium chloride, dibasic sodium phosphate, edetate disodium, sodium metabisulfite, monobasic potassium phosphate, and purified water. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH (5.5 - 7.5); Preservative:benzalkonium chloride (0.004%).
2CLINICAL PHARMACOLOGY
Levobunolol hydrochloride is a noncardioselective beta-adrenoceptor blocking agent, equipotent at both beta
Beta-adrenergic receptor blockade reduces cardiac output in both healthy subjects and patients with heart disease. In patients with severe impairment of myocardial function, beta-adrenergic receptor blockade may inhibit the stimulatory effect of the sympathetic nervous system necessary to maintain adequate cardiac function.
Beta-adrenergic receptor blockade in the bronchi and bronchioles results in increased airway resistance from unopposed parasympathetic activity. Such an effect in patients with asthma or other bronchospastic conditions is potentially dangerous.
Levobunolol hydrochloride ophthalmic solution, USP has been shown to be an active agent in lowering elevated as well as normal intraocular pressure (IOP) whether or not accompanied by glaucoma. Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss.
The onset of action with one drop of levobunolol hydrochloride ophthalmic solution can be detected within one hour after treatment, with maximum effect seen between 2 and 6 hours.
A significant decrease in IOP can be maintained for up to 24 hours following a single dose.
In controlled clinical studies of approximately two years duration, intraocular pressure was well-controlled in approximately 80% of subjects treated with levobunolol hydrochloride ophthalmic solution 0.5% twice a day. The mean IOP decrease from baseline was between 7 mm Hg and 8 mm Hg. No significant effects on pupil size, tear production or corneal sensitivity were observed. Levobunolol hydrochloride ophthalmic solution at the concentrations tested, when applied topically, decreased heart rate and blood pressure in some patients. The IOP-lowering effect of levobunolol hydrochloride ophthalmic solution was well maintained over the course of these studies.
In a three-month clinical study, a single daily application of 0.5% levobunolol hydrochloride ophthalmic solution controlled the IOP of 72% of subjects achieving an overall mean decrease in IOP of 7.0 mm Hg.
The primary mechanism of the ocular hypotensive action of levobunolol hydrochloride in reducing IOP is most likely a decrease in aqueous humor production. Levobunolol hydrochloride ophthalmic solution reduces IOP with little or no effect on pupil size or accommodation.
3INDICATIONS AND USAGE
Levobunolol hydrochloride ophthalmic solution has been shown to be effective in lowering intraocular pressure and may be used in patients with chronic open-angle glaucoma or ocular hypertension.
4CONTRAINDICATIONS
Levobunolol hydrochloride ophthalmic solution is contraindicated in those individuals with bronchial asthma, or with a history of bronchial asthma, or severe chronic obstructive pulmonary disease (see
5WARNINGS
As with other topically applied ophthalmic drugs, levobunolol hydrochloride ophthalmic solution may be absorbed systemically. The same adverse reactions found with systemic administration of beta-adrenergic blocking agents may occur with topical administration. For example, severe respiratory reactions and cardiac reactions, including death due to bronchospasm in patients with asthma, and rarely death in association with cardiac failure, have been reported with topical application of beta-adrenergic blocking agents (see
5.1Cardiac Failure
Sympathetic stimulation may be essential for support of the circulation in individuals with diminished myocardial contractility, and its inhibition by beta-adrenergic receptor blockade may precipitate more severe failure.
5.2In Patients Without a History of Cardiac Failure
Continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first sign or symptom of cardiac failure, levobunolol hydrochloride ophthalmic solution should be discontinued (see
5.3Potentiation of Vascular Insufficiency
Levobunolol hydrochloride ophthalmic solution may potentiate syndromes associated with vascular insufficiency (i.e. Raynaud’s phenomenon), and therefore, should be used with caution in these patients.
5.4Obstructive Pulmonary Disease
PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE (e.g., CHRONIC BRONCHITIS, EMPHYSEMA) OF MILD OR MODERATE SEVERITY, BRONCHOSPASTIC DISEASE OR A HISTORY OF BRONCHOSPASTIC DISEASE (OTHER THAN BRONCHIAL ASTHMA OR A HISTORY OF BRONCHIAL ASTHMA, IN WHICH LEVOBUNOLOL HYDROCHLORIDE OPHTHALMIC SOLUTION IS CONTRAINDICATED, SEE
5.5Major Surgery
The necessity or desirability of withdrawal of beta-adrenergic blocking agents prior to major surgery is controversial. Beta-adrenergic receptor blockade impairs the ability of the heart to respond to beta-adrenergically mediated reflex stimuli. This may augment the risk of general anesthesia in surgical procedures. Some patients receiving beta-adrenergic receptor blocking agents have been subject to protracted severe hypotension during anesthesia. Difficulty in restarting and maintaining the heartbeat has also been reported. For these reasons, in patients undergoing elective surgery, gradual withdrawal of beta-adrenergic blocking agents may be appropriate.
If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of such agonists as isoproterenol, dopamine, dobutamine or levarterenol (see
5.6Diabetes Mellitus
Beta-adrenergic blocking agents should be administered with caution in patients subject to spontaneous hypoglycemia or to diabetic patients (especially those with labile diabetes) who are receiving insulin or oral hypoglycemic agents. Beta-adrenergic blocking agents may mask the signs and symptoms of acute hypoglycemia.
5.7Thyrotoxicosis
Beta-adrenergic blocking agents may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-adrenergic blocking agents which might precipitate a thyroid storm.
5.8Choroidal Detachment
Choroidal detachment after filtration procedures has been reported with the administration of aqueous suppressant therapy.
These products contain sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
6ADVERSE REACTIONS
In clinical trials the use of levobunolol hydrochloride ophthalmic solution has been associated with transient ocular burning and stinging in up to 1 in 3 patients, and with blepharoconjunctivitis in up to 1 in 20 patients. Decreases in heart rate and blood pressure have been reported (see
The following adverse reactions have been reported rarely with the use of levobunolol hydrochloride ophthalmic solution: iridocyclitis, headache, transient ataxia, dizziness, lethargy, urticaria, and pruritus.
Decreased corneal sensitivity has been noted in a small number of patients. Although levobunolol has minimal membrane-stabilizing activity, there remains a possibility of decreased corneal sensitivity after prolonged use.
The following additional adverse reactions have been reported either with levobunolol hydrochloride ophthalmic solution or ophthalmic use of other beta-adrenergic receptor blocking agents:
BODY AS A WHOLE:Headache, asthenia, chest pain. CARDIOVASCULAR:Bradycardia, arrhythmia, hypotension, syncope, heart block, cerebral vascular accident, cerebral ischemia, congestive heart failure, palpitation, cardiac arrest. DIGESTIVE:Nausea, diarrhea. PSYCHIATRIC:Depression, confusion, increase in signs and symptoms of myasthenia gravis, paresthesia. SKIN:Hypersensitivity, including localized and generalized rash, alopecia, Stevens-Johnson Syndrome. RESPIRATORY:Bronchospasm (predominantly in patients with pre-existing bronchospastic disease), respiratory failure, dyspnea, nasal congestion. UROGENITAL:Impotence. ENDOCRINE:Masked symptoms of hypoglycemia in insulin-dependent diabetics (see WARNINGS). SPECIAL SENSES:Signs and symptoms of keratitis or eye allergy, blepharoptosis, visual disturbances including refractive changes (due to withdrawal of miotic therapy in some cases), diplopia, ptosis, and foreign body sensation in eye.
Other reactions associated with the oral use of non-selective adrenergic receptor blocking agents should be considered potential effects with ophthalmic use of these agents.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at
1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
7OVERDOSAGE
No data are available regarding overdosage in humans. Should accidental ocular overdosage occur, flush eye(s) with water or normal saline. If accidentally ingested, efforts to decrease further absorption may be appropriate (gastric lavage). The most common signs and symptoms to be expected with overdosage with administration of a systemic beta-adrenergic blocking agent are symptomatic bradycardia, hypotension, bronchospasm, and acute cardiac failure. Should these symptoms occur, discontinue levobunolol hydrochloride ophthalmic solution therapy and initiate appropriate supportive therapy. The following supportive measures should be considered:
1. Symptomatic bradycardia: Use atropine sulfate intravenously in a dosage of 0.25 mg to 2 mg to induce vagal blockade. If bradycardia persists, intravenous isoproterenol hydrochloride should be administered cautiously. In refractory cases the use of a transvenous cardiac pacemaker should be considered.
2. Hypotension: Use sympathomimetic pressor drug therapy, such as dopamine, dobutamine or levarterenol. In refractory cases the use of glucagon hydrochloride may be useful.
3. Bronchospasm: Use isoproterenol hydrochloride. Additional therapy with aminophylline may be considered.
4. Acute cardiac failure: Conventional therapy with digitalis, diuretics and oxygen should be instituted immediately. In refractory cases the use of intravenous aminophylline is suggested. This may be followed, if necessary, by glucagon hydrochloride which may be useful.
5. Heart block (second or third degree): Use isoproterenol hydrochloride or a transvenous cardiac pacemaker.
8DOSAGE AND ADMINISTRATION
The recommended starting dose is one to two drops of levobunolol hydrochloride ophthalmic solution, 0.5% in the affected eye(s) once a day. In patients with more severe or uncontrolled glaucoma, levobunolol hydrochloride ophthalmic solution, 0.5% can be administered twice a day. As with any new medication, careful monitoring of patients is advised. Dosages above one drop of levobunolol hydrochloride ophthalmic solution, 0.5% twice a day are not generally more effective. If the patient's IOP is not at a satisfactory level on this regimen, concomitant therapy with other ophthalmic IOP-lowering agents can be instituted. Patients should not typically use two or more topical ophthalmic beta-adrenergic blocking agents simultaneously.
9HOW SUPPLIED
Levobunolol hydrochloride ophthalmic solution USP, 0.5% is supplied sterile in a plastic bottle with a controlled drop tip in the following sizes:
NDC 24208-505-05 - 5 mL
NDC 24208-505-10 - 10 mL
NDC 24208-505-15 - 15 mL
Storage:Store between 15°C to 25°C (59°F to 77°F). Protect from light. Replace cap immediately after use.

KEEP OUT OF REACH OF CHILDREN.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
© 2022 Bausch & Lomb Incorporated or its affiliates
Revised: October 2022
9117404
9117504
10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 24208-505-05
Levobunolol
Hydrochloride
Ophthalmic
Solution, USP
0.5% (Sterile)
Hydrochloride
Ophthalmic
Solution, USP
0.5% (Sterile)
FOR OPHTHALMIC
Rx only
5 mL
BAUSCH + LOMB
9564502