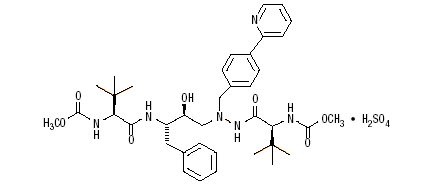
Atazanavir
What is Reyataz (Atazanavir)?
Approved To Treat
Related Clinical Trials
Summary: This research study is designed to test if electrical stimulation of the surface of the brain in the frontal region will help treat depressive symptoms. Participants receive intermittent electrical stimulation to the brain, which involves surgically placing electric leads in between the tough fibrous membrane covering the surface of the brain and the surface of the brain itself. This type of stimu...
Summary: Evaluating the sensitivity and feasibility of using ctDNA assays optimized for detecting very low ctDNA counts from cerebrospinal fluid (CSF) and plasma. The investigators will evaluate the sensitivity of ctDNA from plasma and CSF at baseline (defined as Cycle1 Day1 (C1D1) pre-treatment) and over time in response to treatment with plixorafenib co-administered with cobicistat in BRAF-V600E mutant g...
Summary: The objective of this clinical trial is to determine the effects of a newly formed treatment protocol implemented as telerehabilitation and compare it with conventional physical therapy in patient recovery after Total Knee Arthroplasty. The main questions this study aims to answer are: Does telerehabilitation improve pain, range of motion, and gait as effectively as conventional physical therapy? ...
Related Latest Advances
Brand Information
- REYATAZ is not recommended for use in pediatric patients below the age of 3 months due to the risk of kernicterus
- Use of REYATAZ with ritonavir in treatment-experienced patients should be guided by the number of baseline primary protease inhibitor resistance substitutions
- 200 mg capsule with blue cap and blue body, printed with white ink “BMS 200 mg” on the cap and with white ink “3631” on the body.
- 300 mg capsule with red cap and blue body, printed with white ink “BMS 300 mg” on the cap and with white ink “3622” on the body.
- 50 mg of atazanavir as an oral powder in a packet.
- in patients with previously demonstrated clinically significant hypersensitivity (eg, Stevens-Johnson syndrome, erythema multiforme, or toxic skin eruptions) to any of the components of REYATAZ capsules or REYATAZ oral powder
- when coadministered with drugs that are highly dependent on CYP3A or UGT1A1 for clearance, and for which elevated plasma concentrations of the interacting drugs are associated with serious and/or life-threatening events (see Table 6).
- when coadministered with drugs that are strong inducers of CYP3A due to the potential for loss of therapeutic effect and development of resistance.
- cardiac conduction abnormalities
- rash
- hyperbilirubinemia
- chronic kidney disease
- nephrolithiasis and cholelithiasis
REYATAZ
(atazanavir)
oral powder
- For more information about REYATAZ oral powder, see the Patient Information leaflet.
- REYATAZ oral powder must be mixed with food or liquid. If REYATAZ oral powder is mixed with water, your child must eat food right after taking REYATAZ oral powder.
- REYATAZ oral powder must be taken with ritonavir.
- Talk with your child’s healthcare provider to help decide the best schedule for giving your child REYATAZ oral powder.
- Infants less than 6 months old and who cannot eat solid food or drink from a cup should be given REYATAZ oral powder mixed with infant formula using an oral dosing syringe.
- REYATAZ oral powder that is mixed in infant formula or liquid should not be given using a baby bottle.
- Store REYATAZ oral powder at a temperature of 68°F to 86°F (20°C to 30°C).
- Store REYATAZ oral powder in the original packet. Do not open until ready to use.
- After REYATAZ oral powder is mixed with food or liquid, it may be kept at a temperature of 68°F to 86°F (20°C to 30°C) for up to 1 hour. Take REYATAZ oral powder within 1 hour after mixing with food or liquid.
- Keep REYATAZ oral powder and all medicines out of the reach of children.
- This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
ALERT: Find out about medicines that should NOT be taken with REYATAZ







