Generic Name
Fludeoxyglucose F-18
Brand Names
Fludeoxyglucose, Fludeoxyglucose F18
FDA approval date: August 19, 2005
Classification: Radioactive Diagnostic Agent
Form: Injection
What is Fludeoxyglucose (Fludeoxyglucose F-18)?
Fludeoxyglucose F18 Injection is indicated for positron emission tomography imaging in the following settings: Fludeoxyglucose F18 Injection is indicated for positron emission tomography imaging in the following settings: Oncology: For assessment of abnormal glucose metabolism to assist in the evaluation of malignancy in patients with known or suspected abnormalities found by other testing modalities, or in patients with an existing diagnosis of cancer. Cardiology: For the identification of left ventricular myocardium with residual glucose metabolism and reversible loss of systolic function in patients with coronary artery disease and left ventricular dysfunction, when used together with myocardial perfusion imaging. Neurology: For the identification of regions of abnormal glucose metabolism associated with foci of epileptic seizures. Oncology For assessment of abnormal glucose metabolism to assist in the evaluation of malignancy in patients with known or suspected abnormalities found by other testing modalities, or in patients with an existing diagnosis of cancer. Cardiology For the identification of left ventricular myocardium with residual glucose metabolism and reversible loss of systolic function in patients with coronary artery disease and left ventricular dysfunction, when used together with myocardial perfusion imaging. Neurology For the identification of regions of abnormal glucose metabolism associated with foci of epileptic seizures.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Fludeoxyglucose F 18 (Fludeoxyglucose F-18)
1DESCRIPTION
Fludeoxyglucose F 18 Injection, USP is a positron emitting radiopharmaceutical containing no-carrier added radioactive 2-deoxy-2-[
The active ingredient 2-deoxy-2-[
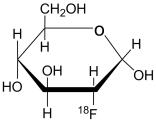
Fludeoxyglucose F 18 Injection, USP is provided as a ready to use isotonic, sterile, pyrogen free, clear, colorless citrate buffered solution. Each mL contains between 0.37 to 3.7 GBq (10.0 – 100 mCi) of 2-deoxy-2-[
1.1Physical Characteristics
Fluorine F 18 decays by positron (β
1.2External Radiation
The specific gamma ray constant for fluorine F 18 is 6.0 R/hr/mCi (0.3 Gy/hr/kB) at 1cm. The half-value layer (HVL) for the 511 keV photons is 4.1 mm lead (Pb). A range of values for the attenuation of radiation results from the interposition of various thickness of Pb. The range of attenuation coefficients for this radionuclide is shown in Table 2. For example, the interposition of an 8.3 mm thickness of Pb, with a coefficient of attenuation of 0.25, will decrease the external radiation by 75%.
For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 3.
2CLINICAL TRIALS
Oncology:1 The efficacy of Fludeoxyglucose F 18 Injection in positron emission tomography cancer imaging was demonstrated in 16 independent literature reports. These studies prospectively evaluated the sensitivity and specificity of Fludeoxyglucose F 18 for detecting malignancies. All these studies had at least 50 patients and used pathology as a standard of truth to compare the results of PET imaging with Fludeoxyglucose F 18 Injection. The studies encompassed a variety of cancers: non-small cell lung cancer, colo-rectal, pancreatic, breast, thyroid, melanoma, Hodgkin’s and non-Hodgkin’s lymphoma, and various types of metastatic cancers to lung, liver, bone, and axillary nodes. The doses in the studies ranged from 200 MBq to 740 MBq with a median and mean dose of 370 MBq.
In these studies the patients had a clinical reason for the evaluation of malignancy (e.g., the patients had an abnormality identified by a prior test and were seeking a diagnosis, or the patients had an existing diagnosis of cancer and were having further work-up or monitoring). None of these studies evaluated the use of Fludeoxyglucose F 18 Injection in routine population screening in which healthy, asymptomatic people are tested for purposes of cancer early detection. The efficacy of Fludeoxyglucose F 18 PET imaging in cancer screening, including its ability to decrease cause-specific mortality, is unknown.
In PET imaging with Fludeoxyglucose F 18 Injection, sensitivity is restricted by the biologic variability of cancer glucose utilization found in individual patients, with different cancers (see Clinical Pharmacology and Pharmacodynamic sections). In the reviewed studies, the sensitivity and specificity varied with the type of cancer, size of cancer, and other clinical parameters. Also, there were false negatives and false positives. Negative PET imaging results with Fludeoxyglucose F 18 Injection do not preclude the diagnosis of cancer and further work-up is indicated. Also, positive PET imaging results with Fludeoxyglucose F 18 Injection cannot replace biopsy to confirm a diagnosis of cancer. There are non-malignant conditions such as fungal infections, inflammatory processes, and benign tumors that had patterns of increased glucose metabolism that give rise to false-positive examinations.
Cardiology:2 The efficacy of Fludeoxyglucose F 18 Injection for cardiac use was demonstrated in ten independent literature reports, which, in general, shared the characteristics summarized below. The studies were prospective and enrolled patients with coronary artery disease and chronic left ventricular systolic dysfunction of a mild to moderate degree. The patients were scheduled to undergo coronary revascularization with either coronary artery bypass surgery or angioplasty. Before revascularization, patients underwent PET imaging with Fludeoxyglucose F 18 Injection and perfusion imaging with other diagnostic radiopharmaceuticals. Doses of Fludeoxyglucose F 18 Injection ranged from 74-370 MBq (2-10 mCi). Segmental, left ventricular, wall-motion assessments of asynergic areas made before revascularization were compared to those made after successful revascularization to identify myocardial segments with functional recovery. Segmental wall motion assessments were made blinded to the results of metabolic/perfusion imaging, and PET image analyses were quantitative.
Left ventricular myocardial segments were predicted to have reversible loss of systolic function if they showed Fludeoxyglucose F 18 accumulation and reduced perfusion (i.e., flow-metabolism mismatch). Conversely, myocardial segments were predicted to have irreversible loss of systolic function if they showed concordant reductions in both Fludeoxyglucose F 18 accumulation and perfusion (i.e., matched defects). Diagnostic performance measures such as sensitivity, specificity, positive predictive value, and negative predictive value were calculated. None of the studies prospectively determined the degree to which mismatch, or the location of mismatch, is associated with improvements in global ventricular function, clinical symptoms, exercise tolerance, or survival.
Findings of flow-metabolism mismatch in a myocardial segment suggest that successful revascularization will restore myocardial function in that segment. However, false-positive tests occur regularly, and the decision to have a patient undergo revascularization should not be based on PET findings alone. Similarly, findings of a matched defect in a myocardial segment suggest that myocardial function will not recover in that segment, even if it is successfully revascularized. However, false-negative tests occur regularly, and the decision to recommend against coronary revascularization, or to recommend a cardiac transplant, should not be based on PET findings alone. The reversibility of segmental dysfunction as predicted with Fludeoxyglucose F 18 PET imaging depends on successful coronary revascularization. Therefore, in patients with a low likelihood of successful revascularization, the diagnostic usefulness of PET imaging with Fludeoxyglucose F 18 Injection is limited.
Epilepsy:3 In a prospective, open label trial, Fludeoxyglucose F 18 Injection was evaluated in 86 patients with epilepsy. Each patient received a dose of Fludeoxyglucose F 18 Injection in the range of 185-370 MBq (5-10 mCi). Demographic characteristics of race and gender are not available. The mean age was 16.4 years (range: 4 months - 58 years; of these, 42 patients were <12 years and 16 patients were <2 years old). Patients had a known diagnosis of complex partial epilepsy and were under evaluation as surgical candidates for treatment of their seizure disorder. Seizure foci had been previously identified on ictal EEGs and sphenoidal EEGs. In 16% (14/87) of patients, the pre-Fludeoxyglucose F 18 Injection findings were confirmed by Fludeoxyglucose F 18; 34% (30/87) of patients, images of Fludeoxyglucose F 18 Injection provided new findings. In 32% (27/87), imaging with Fludeoxyglucose F 18 Injection was not definitive. The influence of these findings on surgical outcome, medical management, or behavior is not known.
Several other studies comparing imaging with Fludeoxyglucose F 18 Injection results to subsphenoidal EEG, MRI and/or surgical findings supported the concept that the degree of hypometabolism corresponds to areas of confirmed epileptogenic foci.
The safety and effectiveness of Fludeoxyglucose F 18 Injection to distinguish idiopathic epileptogenic foci from tumors or other brain lesions that may cause seizures have not been established.
3INDICATIONS AND USAGE
Fludeoxyglucose F 18 Injection, USP is indicated in positron emission tomography (PET) imaging for assessment of abnormal glucose metabolism to assist in the evaluation of malignancy in patients with known or suspected abnormalities found by other testing modalities, or in patients with an existing diagnoses of cancer.
Fludeoxyglucose F 18 Injection, USP is indicated in positron emission tomography (PET) imaging in patients with coronary artery disease and left ventricular dysfunction, when used together with myocardial perfusion imaging, for the identification of left ventricular myocardium with residual glucose metabolism and reversible loss of systolic function.
Fludeoxyglucose F 18 Injection, USP is indicated in positron emission tomography (PET) imaging in patients for the identification of regions of abnormal glucose metabolism associated with foci of epileptic seizures.
4CONTRAINDICATIONS
None known
5WARNINGS
None known
6ADVERSE REACTIONS
The Fludeoxyglucose F 18 Injection safety database for epilepsy included of 374 patients. Of these, 245 were male and 105 were female. For 24 patients, gender was not specified. The mean age was 47.8 years (range under 2 to over 65 years). Eighteen patients were between the age of 0 and 2 years; 42 patients were between the ages of 2 and 21 years; 213 patients were between 21 and 65 years; 98 patients were older than 65 years; and the ages of 3 male patients were not specified. A racial distribution is not available. In this database, adverse drug reactions that required medical intervention were not reported. In a small, 42 patient subset of the 374 patients studied, 4 patients had transient hypotension, 6 had hypo- or hyperglycemia and 3 had transient increases in alkaline phosphatase.
Reviews of the oncology and cardiology literature did not reveal reported adverse reactions.
7DOSAGE AND ADMINISTRATION
The recommended dose of Fludeoxyglucose F 18 Injection for an adult (70 kg) is 185-370 MBq (5-10 mCi), as an intravenous injection for studies of malignancy, cardiology, and epilepsy.
In general, Fludeoxyglucose F 18 Injection should be administered after patients have fasted for 4-6 hours. For cardiac use, Fludeoxyglucose F 18 Injection may be administered either to patients who have fasted or to patients who have received a glucose load (See Patient Preparation section).
The optimum rates of administration and upper safe dose for Fludeoxyglucose F 18 Injection have not been established. The time interval between doses of Fludeoxyglucose F 18 Injection should be long enough to allow substantial decay (physical and biological) of previous administrations.
The final dose for the patient should be calculated using proper decay factors from the time of the end of synthesis (EOS), and measured by a suitable radioactivity calibration system before administration. See decay factors in Table 3.
Patient Preparation: Blood glucose levels should be stabilized before Fludeoxyglucose F 18 Injection is administered. In non-diabetic patients this may be accomplished by fasting 4-6 hours before Fludeoxyglucose F 18 Injection. Diabetic patients may need stabilization of blood glucose on the day preceding and on the day of administration of Fludeoxyglucose F 18 Injection.
For cardiac imaging, administration of Fludeoxyglucose F 18 Injection to fasting patients limits the accumulation of Fludeoxyglucose F 18 to ischemic myocardium. This may make localization of the ischemic region difficult because the surrounding myocardium will not be well visualized. Conversely, administration of Fludeoxyglucose F 18 Injection to patients who have received a glucose load (e.g., 50-75 grams, 1-2 hours before administration of Fludeoxyglucose F 18 Injection) allows the surrounding, non-ischemic myocardium to be seen and facilitates localization of ischemic areas.
Imaging: Optimally, it is recommended that positron emission tomography (PET) imaging be initiated within 40 minutes of administration of Fludeoxyglucose F 18 Injection.
Static emission scans are acquired 30-100 minutes from time of injection.
8OVERDOSAGE
Overdoses of Fludeoxyglucose F 18 Injection have not been reported. See Radiation Dosimetry section for related information.
9DRUG HANDLING
Fludeoxyglucose F 18 Injection, USP, like other parenteral drugs, should be inspected visually for particulate matter and discoloration before administration, whenever solution and container permit. Fludeoxyglucose F 18 Injection preparations containing particulate matter or discoloration should not be administered. They should be disposed of in a safe manner, in compliance with applicable regulations.
Aseptic techniques and effective shielding should be employed in withdrawing doses for administration to patients. Waterproof gloves and effective shielding should be worn when handling the product.
The contents of each vial are sterile and non-pyrogenic. To maintain sterility, aseptic technique must be used during all operations involved in the manipulation and administration of Fludeoxyglucose F 18 Injection, USP.
Fludeoxyglucose F 18 Injection, USP should be used within 12 hours of the end of synthesis (EOS).
As with any other radioactive material, appropriate shielding should be used to avoid unnecessary radiation exposure to the patient, occupational workers, and other persons. Fludeoxyglucose F 18 Injection, USP, like other radioactive drugs, must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Care should be taken to minimize exposure to the patient consistent with proper patient management. Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
10RADIATION DOSIMETRY
The estimated human absorbed radiation doses (rem/mCi) to a 1-year old (9.8 kg), 5-year old (19 kg), 10-year old (32 kg), 15-year old (57 kg), and adult (70 kg) from intravenous administration of Fludeoxyglucose F 18 Injection are shown in Table 4. These estimates were calculated based on human
11REFERENCES
- See March 10, 2000 Federal Register, Docket No. 00N-0553, pp. 12999-13010
- See March 10, 2000 Federal Register, Docket No. 00N-0553, pp. 12999-13010
- See NDA #020306
- Jones, S. C., A. Alavi, Christman, D., Montanez, I., Wolf, A.P. and Reivich, M. (1982).“The Radiation Dosimetry of 2-F-18 fluoro-2-deoxyglucose in Man”. J. Nucl. Me d. 23, 613-617.
- ICRP Publication 53,Volume 18, No. l, 1987, page 76
12HOW SUPPLIED
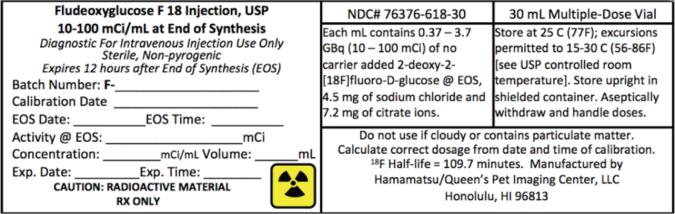
Fludeoxyglucose F 18 Injection, USP is supplied in a multi-dose septum capped 30 mL glass vial containing between 0.37 – 3.7 GBq/mL (10 - 100 mCi/mL), of no carrier added 2-deoxy-2 [
NDC# 76376 - 618- 30
This radiopharmaceutical is licensed by the Nuclear Regulatory Commission, for distribution to entities licensed pursuant to 10 CFR 35.200.
13STORAGE
Store Fludeoxyglucose F 18 Injection, USP at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Store Fludeoxyglucose F 18 Injection, USP multiple-dose vial upright in a lead shielded container.
Store and dispose of Fludeoxyglucose F 18 Injection, USP in accordance with the regulations and a general license, or its equivalent, of an Agreement State or a Licensing State.
13.1Expiration Date and Time
The expiration date and time are provided on the container label. Fludeoxyglucose F 18 Injection, USP should be used within 12 hours from the time of the end of synthesis (EOS).
Caution: Federal Law Prohibits Dispensing Without Prescription
Manufactured and Distributed by:
14PRINCIPAL DISPLAY PANEL
NDC# 76376-618-30
30 mL Multiple-Dose Vial
Fludeoxyglucose F 18 Injection, USP
30 mL Multiple-Dose Vial
Fludeoxyglucose F 18 Injection, USP