Brand Name
Molindone
View Brand InformationFDA approval date: October 17, 2018
Classification: Typical Antipsychotic
Form: Tablet
What is Molindone?
Molindone Hydrochloride Tablets, USP are indicated for the management of schizophrenia. The efficacy of Molindone Hydrochloride Tablets, USP in schizophrenia was established in clinical studies which enrolled newly hospitalized and chronically hospitalized, acutely ill, schizophrenic patients as subjects.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Molindone Hydrochloride (Molindone Hydrochloride)
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis – Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Molindone Hydrochloride Tablets, USP are not approved for the treatment of patients with dementia-related psychosis (see
1DESCRIPTION
Molindone Hydrochloride is a dihydroindolone compound which is not structurally related to the phenothiazines, the butyrophenones or the thioxanthenes.
Molindone Hydrochloride is 3-ethyl-6, 7-dihydro-2-methyl-5-(morpholinomethyl) indol-4(5H)-one hydrochloride. It is a white to off-white or pale-pink crystalline powder, freely soluble in water and alcohol.
Molindone Hydrochloride Tablets, USP contain the following inactive ingredients: alginic acid, calcium sulfate, colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone and sodium starch glycolate.
Colors: 5 mg contains FD&C Yellow #6 Aluminum Lake
- 10 mg contains FD&C Blue #2 Aluminum Lake
- 25 mg contains FD&C Blue #2 Aluminum Lake, FD&C Yellow #6, Aluminum Lake and D&C Yellow #10 Aluminum Lake
Molindone Hydrochloride is represented by the following structural formula:
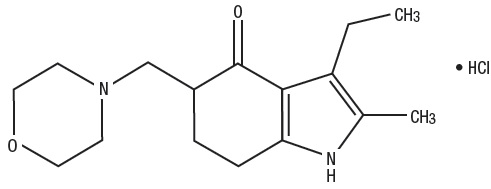
The empirical formula is C
2CLINICAL PHARMACOLOGY
Molindone Hydrochloride has a pharmacological profile in laboratory animals which predominantly resembles that of other antipsychotic agents causing reduction of spontaneous locomotion and aggressiveness, suppression of a conditioned response and antagonism of the bizarre stereotyped behavior and hyperactivity induced by amphetamines. In addition, Molindone Hydrochloride antagonizes the depression caused by the tranquilizing agent tetrabenazine.
In human clinical studies an antipsychotic effect is achieved in the absence of muscle relaxing or incoordinating effects. Based on EEG studies, Molindone Hydrochloride exerts its effect on the ascending reticular activating system.
Human metabolic studies show Molindone Hydrochloride Tablets to be rapidly absorbed and metabolized when given orally. Unmetabolized drug reached a peak blood level at 1.5 hours. Pharmacological effect from a single oral dose persists for 24 to 36 hours. There are 36 recognized metabolites with less than 2 to 3% unmetabolized Molindone Hydrochloride Tablets being excreted in urine and feces.
3INDICATIONS AND USAGE
Molindone Hydrochloride Tablets, USP are indicated for the management of schizophrenia. The efficacy of Molindone Hydrochloride Tablets, USP in schizophrenia was established in clinical studies which enrolled newly hospitalized and chronically hospitalized, acutely ill, schizophrenic patients as subjects.
4CONTRAINDICATIONS
Molindone Hydrochloride Tablets are contraindicated in severe central nervous system depression (alcohol, barbiturates, narcotics, etc.) or comatose states, and in patients with known hypersensitivity to the drug.
5WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis — Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
Molindone Hydrochloride Tablets are not approved for the treatment of patients with dementia-related psychosis (see
5.1Tardive Dyskinesia
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, antipsychotics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to antipsychotic drugs, and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
(For further information about the description of tardive dyskinesia and its clinical detection, please refer to the section on
5.2Falls
Molindone Hydrochloride may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.3Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include, 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
6OVERDOSAGE
Symptomatic, supportive therapy should be the rule.
Gastric lavage is indicated for the reduction of absorption of Molindone Hydrochloride which is freely soluble in water.
Since the adsorption of Molindone Hydrochloride by activated charcoal has not been determined, the use of this antidote must be considered of theoretical value.
Emesis in a comatose patient is contraindicated. Additionally, while the emetic effect of apomorphine is blocked by Molindone Hydrochloride in animals, this blocking effect has not been determined in humans.
A significant increase in the rate of removal of unmetabolized Molindone Hydrochloride from the body by forced diuresis, peritoneal or renal dialysis would not be expected. (Only 2% of a single ingested dose of Molindone Hydrochloride is excreted unmetabolized in the urine). However, poor response of the patient may justify use of these procedures.
While the use of laxatives or enemas might be based on general principles, the amount of unmetabolized Molindone Hydrochloride in feces is less than 1%. Extrapyramidal symptoms have responded to the use of Diphenhydramine (Benadryl
7DOSAGE AND ADMINISTRATION
Initial and maintenance doses of Molindone Hydrochloride Tablets should be individualized.
7.1Initial Dosage Schedule
The usual starting dosage is 50 to 75 mg/day.
—Increase to 100 mg/day in 3 or 4 days.
- —Based on severity of symptomatology, dosage may be titrated up or down depending on individual patient response.
—An increase to 225 mg/day may be required in patients with severe symptomatology.
- Elderly and debilitated patients should be started on lower dosage.
7.2Maintenance Dosage Schedule
- Mild-5 mg to 15 mg three or four times a day.
- Moderate-10 mg to 25 mg three or four times a day.
Severe-225 mg/day may be required.
8HOW SUPPLIED
Molindone Hydrochloride Tablets USP, 5 mg are light orange to orange, oval-shaped tablets, debossed "
Bottles of 100 NDC 42806-336-01
Molindone Hydrochloride Tablets USP, 10 mg are light blue to blue, oval-shaped tablets, debossed "
Bottles of 100 NDC 42806-337-01
Molindone Hydrochloride Tablets USP, 25 mg are light green to green, oval-shaped tablet, debossed "
Bottles of 100 NDC 42806-338-01
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP TIGHTLY CLOSED
*Benadryl is a registered trademark of Warner-Lambert.
†Symmetrel is a registered trademark of Endo Pharmaceuticals Inc.
‡Artane is a registered trademark of Lederle Laboratories.
§Cogentin is a registered trademark of Merck & Co., Inc.
¶Akineton is a registered trademark of Knoll Laboratories.
Distributed by:
Epic Pharma, LLC
Laurelton NY, 11413
Rev. 11-2024-00
MF336REV11/24
OE2798
9PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Molindone 5 mg 100ct

10PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Molindone 10 mg 100ct

11PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Molindone 25 mg 100ct
