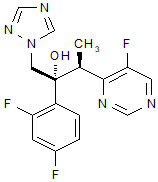
Vfend
What is Vfend (Voriconazole)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The purpose of this clinical trial is to compare drug combinations to learn which drugs work best to prevent graft-versus-host-disease (GVHD) in people who have received a stem cell transplant. The source of stem cells is from someone who is not related and has a different blood cell type than the study participant. The researchers will compare the new drug combination to a standard drug combinati...
Summary: The goal of this interventional clinical trial is to determine if low doses of gentle chemotherapy after bone marrow transplant may prevent relapse and promote an increase in survival and decrease in side effects in participants with acute myeloid leukemia and myelodysplastic syndromes. The main question it aims to answer is whether or not providing a new, gentler way of administering chemotherapy...
Summary: The phase 1 portion of the study is designed to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of TLC-6740 after single- and multiple-ascending doses in healthy subjects. The phase 1b portion of the study is designed to assess the safety, tolerability, and PK of TLC-6740 in subjects with obesity, with or without type 2 diabetes mellitus.
Related Latest Advances
Brand Information
- VFEND is contraindicated in patients with known hypersensitivity to voriconazole or its excipients. There is no information regarding cross-sensitivity between VFEND (voriconazole) and other azole antifungal agents. Caution should be used when prescribing VFEND to patients with hypersensitivity to other azoles.
- Coadministration of pimozide, quinidine or ivabradine with VFEND is contraindicated because increased plasma concentrations of these drugs can lead to QT prolongation and rare occurrences of
- Coadministration of VFEND with sirolimus is contraindicated because VFEND significantly increases sirolimus concentrations
- Coadministration of VFEND with rifampin, carbamazepine, long-acting barbiturates or St. John's Wort is contraindicated because these drugs are likely to decrease plasma voriconazole concentrations significantly
- Coadministration of standard doses of voriconazole with efavirenz doses of 400 mg every 24 hours or higher is contraindicated, because efavirenz significantly decreases plasma voriconazole concentrations in healthy subjects at these doses. Voriconazole also significantly increases efavirenz plasma concentrations
- Coadministration of VFEND with high-dose ritonavir (400 mg every 12 hours) is contraindicated because ritonavir (400 mg every 12 hours) significantly decreases plasma voriconazole concentrations. Coadministration of voriconazole and low-dose ritonavir (100 mg every 12 hours) should be avoided, unless an assessment of the benefit/risk to the patient justifies the use of voriconazole
- Coadministration of VFEND with rifabutin is contraindicated since VFEND significantly increases rifabutin plasma concentrations and rifabutin also significantly decreases voriconazole plasma concentrations
- Coadministration of VFEND with ergot alkaloids (ergotamine and dihydroergotamine) is contraindicated because VFEND may increase the plasma concentration of ergot alkaloids, which may lead to ergotism
- Coadministration of VFEND with naloxegol is contraindicated because VFEND may increase plasma concentrations of naloxegol which may precipitate opioid withdrawal symptoms
- Coadministration of VFEND with tolvaptan is contraindicated because VFEND may increase tolvaptan plasma concentrations and increase risk of adverse reactions
- Coadministration of VFEND with venetoclax at initiation and during the ramp-up phase is contraindicated in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) due to the potential for increased risk of tumor lysis syndrome
- Coadministration of VFEND with lurasidone is contraindicated since it may result in significant increases in lurasidone exposure and the potential for serious adverse reactions
- Coadministration of VFEND with finerenone is contraindicated since it may result in significant increases in finerenone exposure and the potential for serious adverse reactions
- Advise patients of the risk of photosensitivity (with or without concomitant methotrexate), accelerated photoaging, and skin cancer.
- Advise patients that VFEND can cause serious photosensitivity and to immediately contact their healthcare provider for new or worsening skin rash.
- Advise patients to avoid exposure to direct sun light and to use measures such as protective clothing and sunscreen with high sun protection factor (SPF).
- Advise female patients of the potential risks to a fetus.
- Advise females of reproductive potential to use effective contraception during treatment with VFEND.
(voriconazole) for injection
200 mg* of voriconazole
No Preservatives

200 mg* of voriconazole
rubber latex
No Preservatives


