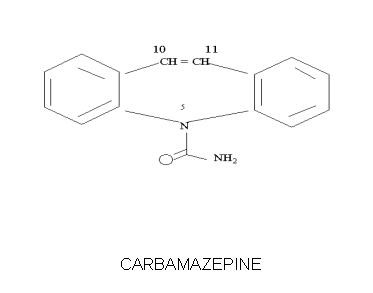
Carbamazepine
What is Carbatrol (Carbamazepine)?
Living with epilepsy or chronic nerve pain can deeply affect a person’s sense of safety, focus, and independence. Seizures or sudden episodes of pain can interrupt daily life and make even ordinary tasks unpredictable. Carbatrol (carbamazepine) is a medication that helps bring control and stability back into the lives of people affected by these conditions.
Carbatrol is an anticonvulsant and mood-stabilizing medication used to treat seizure disorders, certain types of nerve pain, and bipolar disorder. It belongs to a drug class known as dibenzazepine anticonvulsants. Carbatrol is an extended-release formulation of carbamazepine, meaning it delivers medication slowly throughout the day for steady, long-term control of symptoms. It has been a well-established, first-line therapy for decades, trusted for its effectiveness in stabilizing abnormal brain activity and improving quality of life.
What does Carbatrol do?
Carbatrol is primarily prescribed to treat epilepsy, a neurological condition characterized by recurrent seizures caused by sudden bursts of electrical activity in the brain. By helping regulate this electrical activity, Carbatrol reduces the frequency and severity of seizures, allowing patients to live with fewer interruptions and greater confidence.
It’s also used to relieve trigeminal neuralgia, a chronic pain disorder affecting the face. People with this condition experience sudden, intense facial pain triggered by simple actions like chewing or talking. Carbatrol helps by calming the overactive nerves responsible for these painful episodes.
Additionally, Carbatrol can help stabilize mood swings in bipolar disorder, particularly during manic episodes. It supports emotional balance and helps prevent extreme highs or lows.
Clinical studies and long-term use have shown that carbamazepine effectively reduces seizure frequency and nerve pain intensity in most patients, making it one of the most widely prescribed and trusted anticonvulsants worldwide (NIH, 2024).
How does Carbatrol work?
Carbatrol works by stabilizing the electrical signals in the brain and nerves. In simple terms, it prevents nerve cells from becoming overly excited, a common trigger for seizures and certain types of nerve pain.
It does this by blocking sodium channels in nerve cells. These channels are responsible for transmitting electrical impulses. When too many impulses fire at once, it can lead to seizures or pain signals. By slowing the flow of sodium into these cells, Carbatrol calms overactive nerves and helps them function more normally.
This mechanism is also believed to help regulate mood in people with bipolar disorder by balancing neurotransmitter activity in the brain. Clinically, this stabilization of nerve and brain activity reduces the likelihood of seizures, eases nerve pain, and promotes emotional stability helping patients lead safer, more comfortable lives.
Carbatrol side effects
Like all prescription medications, Carbatrol can cause side effects. Most are mild and tend to improve as the body adjusts to the medication. However, it’s important to understand both the common and serious reactions.
Common side effects may include:
- Dizziness or drowsiness
- Nausea or vomiting
- Unsteadiness or coordination issues
- Blurred or double vision
- Mild skin rash
Serious side effects (rare but important to recognize):
- Severe allergic skin reactions (such as Stevens-Johnson syndrome)
- Unusual bleeding or bruising (possible blood cell changes)
- Signs of infection (fever, sore throat)
- Yellowing of the skin or eyes (liver problems)
- Confusion, slurred speech, or unsteady movements (possible toxicity)
Because of potential effects on blood cells and liver function, doctors typically perform regular blood tests during treatment to ensure the medication remains safe and effective.
Who should avoid Carbatrol:
People with a known allergy to carbamazepine or tricyclic antidepressants, or those with a history of bone marrow suppression, should not take this medication. It should also be used cautiously in patients with liver, kidney, or heart conditions.
Seek immediate medical attention for signs of allergic reaction, severe rash, or symptoms such as weakness, fever, or dark urine. While these effects are uncommon, prompt attention ensures safe, continued treatment.
Carbatrol dosage
Carbatrol is an extended-release capsule taken orally for steady symptom control with fewer daily doses. Swallow capsules whole; do not crush or chew. It can be taken with or without food. Dosage is individualized, starting low and increasing gradually to minimize side effects.
Due to Carbatrol’s impact on the liver and blood cells, healthcare providers monitor complete blood counts (CBCs), liver function tests (LFTs), and sodium levels to maintain safe and therapeutic ranges.
Older adults or those with liver or kidney impairment may require lower doses or closer monitoring. Abruptly stopping Carbatrol can cause withdrawal seizures or symptom rebound; therefore, all medication changes must be professionally supervised.
Does Carbatrol have a generic version?
Yes. The active ingredient in Carbatrol, carbamazepine, is available as a generic medication approved by the U.S. Food and Drug Administration (FDA). Generic carbamazepine formulations including immediate-release and extended-release form are clinically equivalent to Carbatrol in safety, strength, and effectiveness.
Carbamazepine, also known as Tegretol or Equetro, may be switched between brand and generic versions under medical supervision for consistent symptom control.
Conclusion
Carbatrol (carbamazepine) is a proven and widely used medication that helps control seizures, relieve nerve pain, and stabilize mood. By calming overactive nerve signals in the brain, it allows patients to regain control over their health and daily routines.
Though requiring careful monitoring and potential side effects, Carbatrol is a highly effective long-term epilepsy treatment. Most patients tolerate it well, achieving significant stability and comfort. Success hinges on consistent use, open communication with your provider, and regular monitoring for safety and effectiveness. With proper care, Carbatrol helps patients live more confident, seizure-free, and pain-controlled lives.
References
- U.S. Food and Drug Administration (FDA). (2024). Carbamazepine prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Carbamazepine (oral route) medication overview. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Carbamazepine: Drug uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Anticonvulsant therapies and mechanism of action. Retrieved from https://www.nih.gov
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Should not be used
with other carbamazepine
containing products.
Should not be used
with other carbamazepine
containing products.
Should not be used
with other carbamazepine
containing products.
