Uplizna
What is Uplizna (Inebilizumab)?
Living with a chronic autoimmune disease that attacks the body’s nervous system can be frightening and unpredictable. Conditions like neuromyelitis optica spectrum disorder (NMOSD) can cause sudden vision loss, paralysis, or severe pain, dramatically affecting independence and quality of life. Uplizna (inebilizumab-cdon) is designed to help change that outlook by reducing relapses and protecting the nervous system from further damage.
Uplizna is a prescription biologic medication approved by the U.S. Food and Drug Administration (FDA) in 2020. It belongs to a class of drugs known as monoclonal antibodies, which are specialized proteins that target specific immune cells involved in disease activity. Uplizna is not a cure for NMOSD, but it is a long-term maintenance therapy that helps control the disease and prevent new attacks. By decreasing inflammation and immune activity in the nervous system, it helps patients maintain vision, mobility, and independence.
What does Uplizna do?
Uplizna is used to treat adults with neuromyelitis optica spectrum disorder (NMOSD) who are positive for the aquaporin-4 (AQP4) antibody, a marker found in most people with this condition.
NMOSD is a rare autoimmune disorder in which the immune system mistakenly attacks optic nerves and the spinal cord, leading to episodes of inflammation called relapses. Each relapse can cause severe and sometimes permanent damage, such as blindness or paralysis.
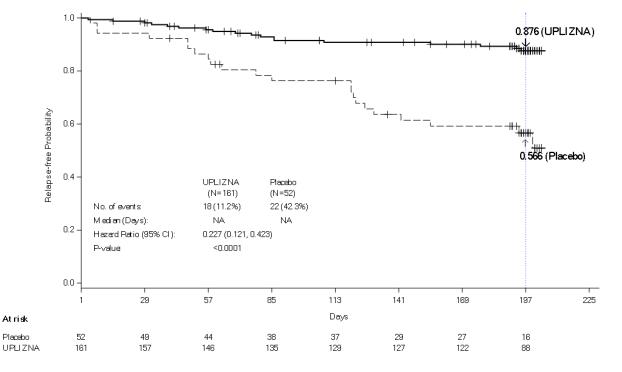
Uplizna works by reducing the number of these relapses, helping prevent further nerve damage. In clinical trials, patients treated with Uplizna experienced about a 77% reduction in the risk of relapse compared to those given a placebo (FDA, 2020). Many patients report fewer flare-ups, improved stability, and better long-term outcomes.
This medication is considered a specialized or targeted therapy, typically prescribed by neurologists who manage autoimmune or demyelinating diseases. It is often chosen for patients who have tested positive for the AQP4 antibody and are looking for a reliable, infrequently dosed treatment to maintain remission.
How does Uplizna work?
Uplizna works by targeting and depleting specific immune cells called B cells, which play a central role in NMOSD. These B cells produce antibodies including the harmful AQP4 antibodies that attack the nervous system.
The drug’s active ingredient, inebilizumab, is a monoclonal antibody that binds to a protein called CD19, found on the surface of B cells. Once attached, it helps the immune system remove these cells from circulation. By reducing the number of B cells, Uplizna lowers the production of damaging antibodies and decreases inflammation in the optic nerves and spinal cord.
This mechanism is crucial because preventing attacks is the best way to avoid long-term disability. Unlike medications that only reduce symptoms during an attack, Uplizna acts proactively to protect the nervous system from further damage. Its effects are long-lasting, allowing for treatment only a few times a year while maintaining steady disease control.
Uplizna side effects
As with any powerful immune therapy, Uplizna can cause side effects, though many are mild and manageable.
Common side effects include:
- Headache
- Fatigue
- Nausea
- Back pain
- Urinary tract infections
- Pain or redness at the infusion site
Serious but less common side effects:
- Infusion reactions: These may include fever, chills, rash, or shortness of breath during or after treatment. Most are mild and managed by slowing the infusion or giving medication beforehand.
- Infections: Because Uplizna lowers certain immune cells, it may slightly increase the risk of infections, such as respiratory or urinary tract infections.
- Reduced immunoglobulin levels: Over time, some patients may have lower antibody levels, which are monitored through blood tests.
Who should avoid Uplizna:
Uplizna should not be used by people with active infections, hepatitis B, or known allergies to inebilizumab or its ingredients. Before starting treatment, doctors typically screen for infections such as hepatitis or tuberculosis to ensure it’s safe to begin therapy.
Patients should seek immediate medical attention if they experience symptoms such as severe rash, difficulty breathing, persistent fever, or unexplained weakness. Regular checkups and lab monitoring help detect and manage side effects early, keeping treatment safe and effective.
Uplizna dosage
Uplizna is an intravenous (IV) infusion given by a healthcare professional. Initial infusions are two weeks apart, then every six months. Each 90-minute session in a clinic may include premedication to prevent reactions.
During Uplizna treatment, doctors monitor blood counts, liver function, and immunoglobulin levels. Vaccinations should be completed before therapy due to infection risk. Dosing is consistent for all adults, but older or comorbid patients may require additional monitoring.
Does Uplizna have a generic version?
As of 2025, Uplizna (inebilizumab-cdon) does not have an FDA-approved generic version. It is available only as the brand-name product, developed by Horizon Therapeutics. However, international versions may exist in other markets.
Uplizna, a biologic, has no currently available biosimilar. Future alternatives will be biosimilars, not generics, meaning they will be highly similar with no meaningful differences in safety, purity, or effectiveness. Patients can explore manufacturer financial assistance programs.
Conclusion
Uplizna represents a major advancement in the treatment of neuromyelitis optica spectrum disorder. By targeting the immune cells responsible for nerve inflammation, it helps prevent relapses that can lead to blindness or paralysis giving patients renewed hope for a stable and independent life.
With medical supervision and periodic infusions, Uplizna offers long-lasting, convenient, and powerful disease control for NMOSD. Most patients tolerate it well with proper monitoring. It provides a chance to regain control, reduce uncertainty, and face the future with confidence.
References
- U.S. Food and Drug Administration (FDA). (2020). Uplizna (inebilizumab-cdon) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Inebilizumab (intravenous route) drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Inebilizumab injection: Uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Advances in treatment of neuromyelitis optica spectrum disorder. Retrieved from https://www.nih.gov
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- A history of a life-threatening infusion reaction to UPLIZNA
- Active hepatitis B infection
- Active or untreated latent tuberculosis
- Infusion Reactions
- Infections
- Reduction in Immunoglobulins
- A history of one or more relapses that required rescue therapy within the year prior to screening, or 2 or more relapses that required rescue therapy in 2 years prior to screening.
- Expanded Disability Status Scale (EDSS) score of 7.5 or less. Patients with an EDSS score of 8.0 were eligible if they were deemed capable of participating.
- Patients were excluded if previously treated with immunosuppressant therapies within an interval specified for each such therapy.