Brand Name
Givlaari
Generic Name
Givosiran
View Brand Information FDA approval date: December 12, 2019
Classification: Small Interfering RNA
Form: Injection
What is Givlaari (Givosiran)?
GIVLAARI is indicated for the treatment of adults with acute hepatic porphyria . GIVLAARI is an aminolevulinate synthase 1-directed small interfering RNA indicated for the treatment of adults with acute hepatic porphyria .
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
ELEVATE, a Global Observational Longitudinal Prospective Registry of Patients With Acute Hepatic Porphyria (AHP)
Summary: This global patient registry is being conducted to characterize the natural history and real-world clinical management of patients with AHP, and to further characterize the real-world safety and effectiveness of givosiran and other approved AHP therapies.
Related Latest Advances
Brand Information
GIVLAARI (givosiran sodium)
1INDICATIONS AND USAGE
GIVLAARI is indicated for the treatment of adults with acute hepatic porphyria (AHP).
2DOSAGE FORMS AND STRENGTHS
Injection: 189 mg/mL clear, colorless-to-yellow solution in a single-dose vial
3CONTRAINDICATIONS
GIVLAARI is contraindicated in patients with known severe hypersensitivity to givosiran. Reactions have included anaphylaxis
4ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Anaphylactic Reaction
- Transaminase Elevations
- Serum Creatinine Increase
- Injection Site Reactions
- Blood Homocysteine Increased
- Pancreatitis
4.1Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the pivotal placebo-controlled, double-blind study (ENVISION), 48 patients received 2.5 mg/kg GIVLAARI and 46 patients received placebo, administered once monthly via subcutaneous injection for up to 6 months. Patients received GIVLAARI for a median of 5.5 months (range 2.7-6.4 months). Of these, 47 patients received ≥5 months of treatment. The most frequently occurring (≥20% incidence) adverse reactions reported in patients treated with GIVLAARI were nausea (27%) and injection site reactions (25%). Permanent discontinuation occurred in one patient due to elevated transaminases.
Adverse reactions observed at a lower frequency occurring in placebo-controlled and open-label clinical studies included anaphylactic reaction (one patient, 0.9%) and hypersensitivity (one patient, 0.9%).
In the ENVISION study, during the open label extension, adverse reactions of blood homocysteine increased were reported in 15 of 93 (16%) patients treated with GIVLAARI
4.2Immunogenicity
As with all oligonucleotides, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
In placebo-controlled and open-label clinical studies, 1 of 111 patients with AHP (0.9%) developed treatment-emergent anti-drug antibodies (ADA) during treatment with GIVLAARI. No clinically significant differences in the clinical efficacy, safety, pharmacokinetic, or pharmacodynamic profiles of GIVLAARI were observed in the patient who tested positive for anti-givosiran antibodies.
4.3Postmarketing Experience
The following additional adverse reactions have been reported during post-approval use. Because these events are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: Acute pancreatitis
5DESCRIPTION
GIVLAARI is an aminolevulinate synthase 1-directed small interfering RNA (siRNA), covalently linked to a ligand containing three N-acetylgalactosamine (GalNAc) residues to enable delivery of the siRNA to hepatocytes.
The structural formulas of the givosiran drug substance in its sodium form, and the ligand (L96), are presented below.
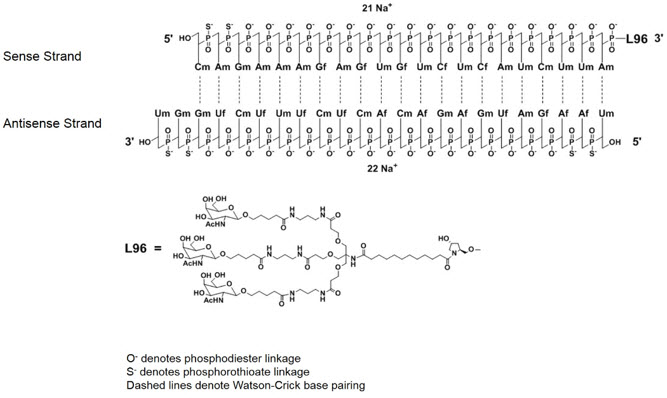
Abbreviations: Af = adenine 2'-F ribonucleoside; Cf = cytosine 2'-F ribonucleoside; Uf = uracil 2'-F ribonucleoside; Am = adenine 2'-OMe ribonucleoside; Cm = cytosine 2'-OMe ribonucleoside; Gf = guanine 2'-F ribonucleoside; Gm = guanine 2'-OMe ribonucleoside; Um = uracil 2'-OMe ribonucleoside; L96 = triantennary GalNAc (N-acetylgalactosamine)
GIVLAARI is supplied as a sterile, preservative-free, 1-mL colorless-to-yellow solution for subcutaneous injection containing 189 mg givosiran in a single-dose, 2-mL Type 1 glass vial with a fluoropolymer-coated rubber stopper and a flip-off aluminum seal. GIVLAARI is available in cartons containing one single-dose vial each
The molecular formula of givosiran sodium is C
The molecular formula of givosiran (free acid) is C
6CLINICAL STUDIES
The efficacy of GIVLAARI in patients with acute hepatic porphyria was evaluated in the ENVISION trial (NCT03338816), a randomized, double-blind, placebo-controlled, multinational study.
ENVISION enrolled 94 patients with acute hepatic porphyria (AHP) (89 patients with AIP, 2 patients with variegate porphyria [VP], 1 patient with hereditary coproporphyria [HCP], and 2 patients with no identified mutation). Eligible patients were randomized 1:1 to receive once monthly subcutaneous injections of GIVLAARI 2.5 mg/kg or placebo during the 6-month double-blind period. In this study, inclusion criteria specified a minimum of 2 porphyria attacks requiring hospitalization, urgent healthcare visit, or intravenous hemin administration at home in the 6 months prior to study entry. After the 6 month treatment period patients were enrolled in an open label extension period for up to 30 months. Ninety-three patients were enrolled in the open label extension period. Hemin use during the study was permitted for the treatment of acute porphyria attacks.
The median age of patients studied was 37.5 years (range 19 to 65 years), 89% of patients were female, and 78% were white. GIVLAARI and placebo arms were balanced with respect to historical porphyria attack rate, hemin prophylaxis prior to study entry, use of opioid medications, and patient-reported measures of pain symptoms between attacks.
Efficacy in the 6-month double-blind period was measured by the rate of porphyria attacks that required hospitalizations, urgent healthcare visit, or intravenous hemin administration at home.
Efficacy results for GIVLAARI are provided in Table 3. On average, AHP patients on GIVLAARI experienced 70% (95% CI: 60%, 80%) fewer porphyria attacks compared to placebo.
GIVLAARI also resulted in a reduction in hemin use, urinary ALA, and urinary PBG.
7PATIENT COUNSELING INFORMATION
Advise patients of the potential risks of GIVLAARI treatment:
- Anaphylactic Reaction: Inform patients about the risk and possible symptoms of severe hypersensitivity reactions that could occur [see .
- Hepatic Toxicity: Inform patients that transaminase elevations may occur, and that laboratory testing will be conducted in the first 6 months of treatment and as clinically indicated thereafter [see .
- Renal Toxicity: Inform patients that increases in serum creatinine and decreases in eGFR have been reported and that laboratory testing will be conducted as clinically indicated [see .
- Injection Site Reactions: Inform patients of the signs and symptoms of injection site reactions (examples include redness, pain, itching, rash, discoloration, or localized swelling) [see
- Blood Homocysteine Increased: Inform patients that increases in blood homocysteine levels have been reported when using GIVLAARI, and that laboratory testing will be conducted prior to and during treatment with GIVLAARI. Vitamin supplementation may be considered for elevated blood homocysteine levels [see
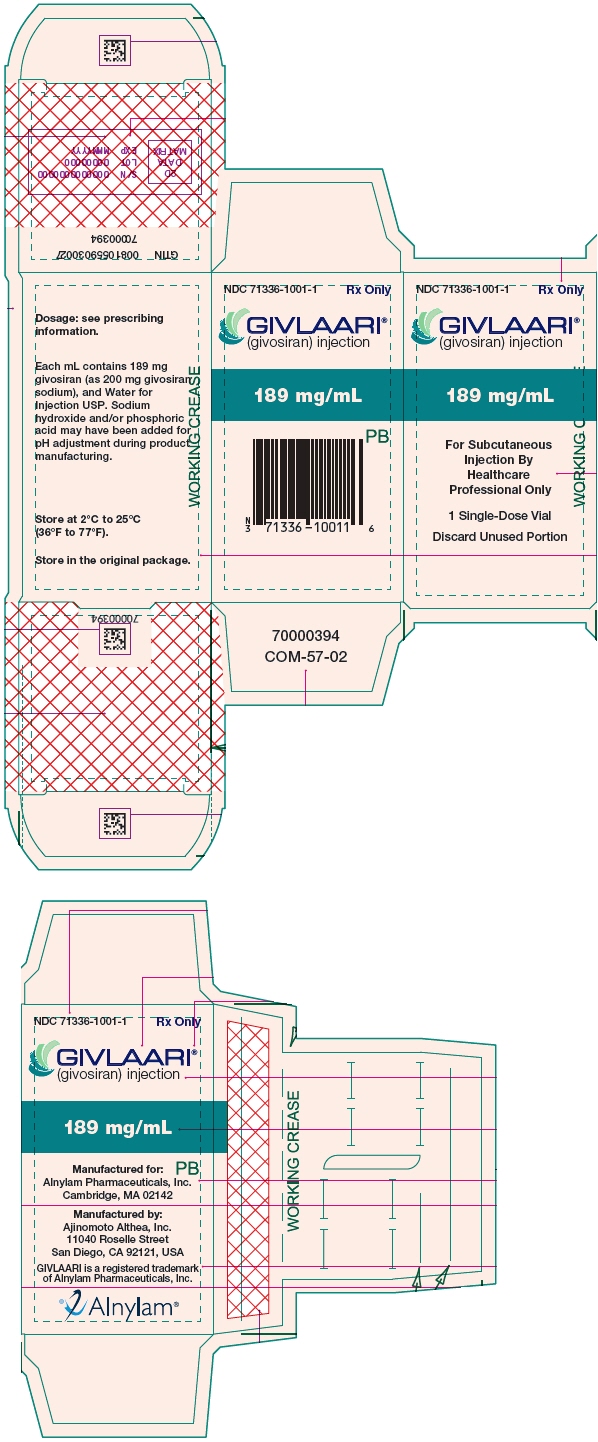
8PRINCIPAL DISPLAY PANEL - 189 mg/mL Vial Carton
NDC 71336-1001-1
GIVLAARI
189 mg/mL
For Subcutaneous
1 Single-Dose Vial
Discard Unused Portion