Brand Name
Somatuline
Generic Name
Lanreotide Acetate
View Brand Information FDA approval date: November 14, 2007
Classification: Somatostatin Analog
Form: Injection
What is Somatuline (Lanreotide Acetate)?
Lanreotide Injection is a somatostatin analog indicated for: the long-term treatment of acromegalic patients who have had an inadequate response to or cannot be treated with surgery and/or radiotherapy.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
SOMATULINE DEPOT (lanreotide acetate)
1DOSAGE FORMS AND STRENGTHS
Injection: 60 mg/0.2 mL, 90 mg/0.3 mL, and 120 mg/0.5 mL sterile, single-dose, prefilled syringes fitted with an automatic safety system (attached retractable needle and needle guard). The prefilled syringes contain a white to pale yellow, semi-solid formulation.
2CONTRAINDICATIONS
SOMATULINE DEPOT is contraindicated in patients with history of a hypersensitivity to lanreotide. Allergic reactions (including angioedema and anaphylaxis) have been reported following administration of lanreotide
3ADVERSE REACTIONS
The following adverse reactions to SOMATULINE DEPOT are discussed in greater detail in other sections of the labeling:
- Cholelithiasis and Complications of Cholelithiasis
- Hyperglycemia and Hypoglycemia
- Cardiovascular Abnormalities
- Thyroid Function Abnormalities
- Steatorrhea and Malabsorption of Dietary Fats
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
3.2Immunogenicity
As with all peptides, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to lanreotide in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Laboratory investigations of acromegalic patients treated with SOMATULINE DEPOT in clinical studies show that the percentage of patients with putative antibodies at any time point after treatment is low (less than 1% to 4% of patients in specific studies whose antibodies were tested). The antibodies did not appear to affect the efficacy or safety of SOMATULINE DEPOT.
In Study 3, development of anti-lanreotide antibodies was assessed using a radioimmunoprecipitation assay. In patients with GEP NETs receiving SOMATULINE DEPOT, the incidence of anti-lanreotide antibodies was 4% (3 of 82) at 24 weeks, 10% (7 of 67) at 48 weeks, 11% (6 of 57) at 72 weeks, and 10% (8 of 84) at 96 weeks. Assessment for neutralizing antibodies was not conducted. In Study 4, less than 2% (2 of 108) of the patients treated with SOMATULINE DEPOT developed anti-lanreotide antibodies.
3.3Postmarketing Experience
The following adverse reactions have been identified during post-approval use of SOMATULINE DEPOT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal disorders: pancreatic exocrine insufficiency
Hepatobiliary: steatorrhea; cholecystitis, cholangitis, pancreatitis, which have sometimes required cholecystectomy
Hypersensitivity: angioedema and anaphylaxis
Injection site reactions: injection site abscess
Hepatobiliary: steatorrhea; cholecystitis, cholangitis, pancreatitis, which have sometimes required cholecystectomy
Hypersensitivity: angioedema and anaphylaxis
Injection site reactions: injection site abscess
4DESCRIPTION
SOMATULINE DEPOT (lanreotide) Injection 60 mg/0.2 mL, 90 mg/0.3 mL, and 120 mg/0.5 mL is a prolonged-release formulation for deep subcutaneous injection. It contains the drug substance lanreotide acetate, a synthetic octapeptide with a biological activity similar to naturally occurring somatostatin, water for injection and acetic acid (for pH adjustment).
SOMATULINE DEPOT is available as sterile, ready-to-use, single-dose prefilled syringes containing lanreotide acetate supersaturated bulk solution of 24.6% w/w lanreotide base.
Lanreotide acetate is a synthetic cyclical octapeptide analog of the natural hormone, somatostatin. Lanreotide acetate is chemically known as [cyclo S-S]-3-(2-naphthyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-L-threoninamide, acetate salt. Its molecular weight is 1096.34 (base) and its amino acid sequence is:
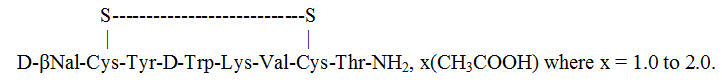
The SOMATULINE DEPOT in the prefilled syringe is a white to pale yellow, semi-solid formulation.
5HOW SUPPLIED/STORAGE AND HANDLING
SOMATULINE DEPOT is supplied in strengths of 60 mg/0.2 mL, 90 mg/0.3 mL, and 120 mg/0.5 mL as a white to pale yellow, semi-solid formulation in a single, sterile, prefilled, ready-to-use, polypropylene syringe fitted with an automatic safety system, a bromobutyl rubber plunger stopper and a 20 mm attached needle covered by a plastic cap.
Each prefilled syringe is placed in a plastic tray, sealed in a laminated pouch and packed in a carton.
6PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
7PRINCIPAL DISPLAY PANEL - 60 mg/0.2 mL Syringe Carton - NDC 15054-1060-4
Rx only
Somatuline
(lanreotide) Injection
60 mg/0.2 mL
(lanreotide) Injection
60 mg/0.2 mL
NDC 15054 1060 4
For deep subcutaneous injection
For single use only. Discard unused portion.
Leave at room temperature for 30 minutes before administration.
CONTENTS: This box contains one (1) pre-filled syringe.
60 mg/0.2 mL
IPSEN

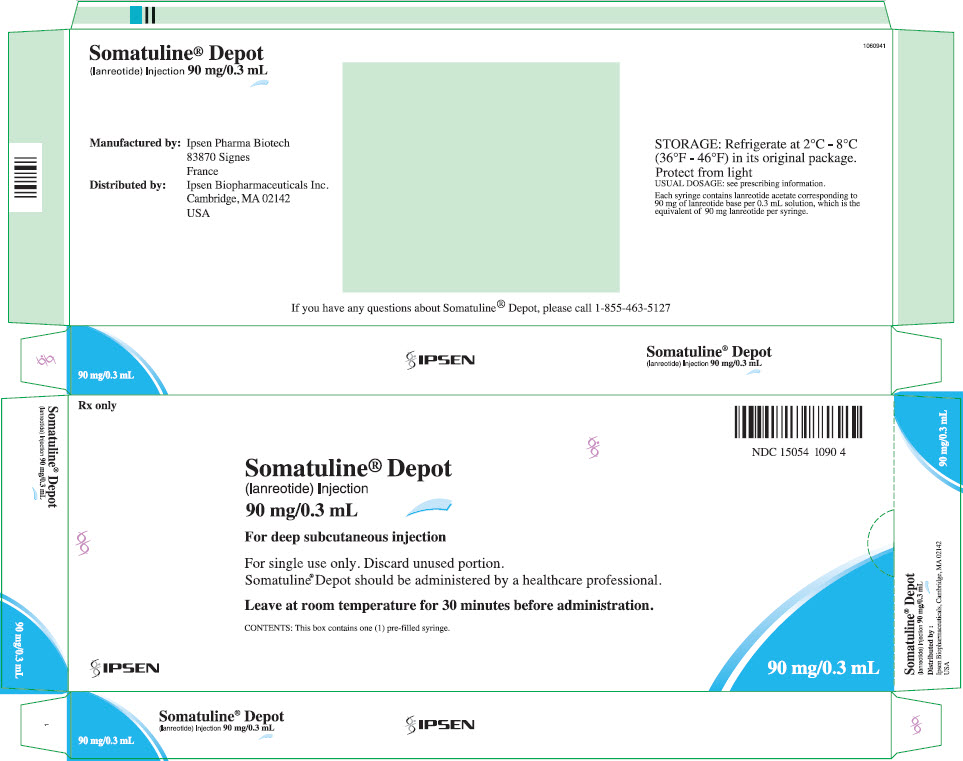
8PRINCIPAL DISPLAY PANEL - 90 mg/0.3 mL Syringe Carton - NDC 15054-1090-4
Rx only
Somatuline
(lanreotide) Injection
90 mg/0.3 mL
(lanreotide) Injection
90 mg/0.3 mL
NDC 15054 1090 4
For deep subcutaneous injection
For single use only. Discard unused portion.
Leave at room temperature for 30 minutes before administration.
CONTENTS: This box contains one (1) pre-filled syringe.
90 mg/0.3 mL
IPSEN
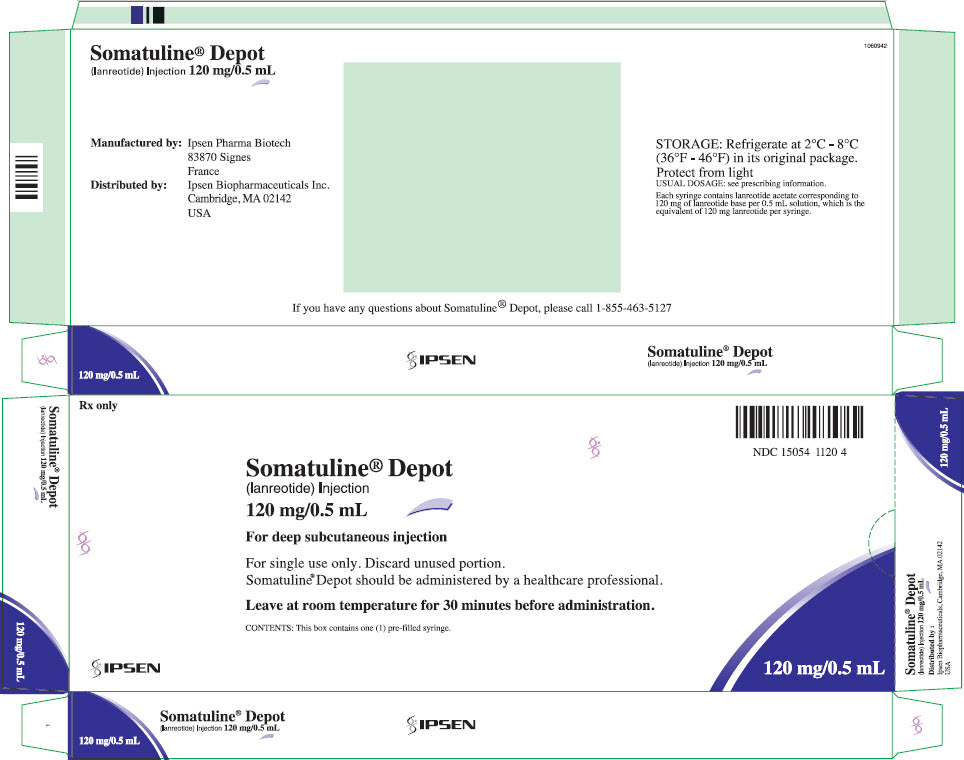
9PRINCIPAL DISPLAY PANEL - 120 mg/0.5 mL Syringe Carton - NDC 15054-1120-4
Rx only
Somatuline
(lanreotide) Injection
120 mg/0.5 mL
(lanreotide) Injection
120 mg/0.5 mL
NDC 15054 1120 4
For deep subcutaneous injection
For single use only. Discard unused portion.
Leave at room temperature for 30 minutes before administration.
CONTENTS: This box contains one (1) pre-filled syringe.
120 mg/0.5 mL
IPSEN