Brand Name
Arikayce
Generic Name
Amikacin
View Brand Information FDA approval date: December 24, 2013
Classification: Aminoglycoside Antibacterial
Form: Injection, Suspension
What is Arikayce (Amikacin)?
Amikacin Sulfate Injection USP is indicated in the short-term treatment of serious infections due to susceptible strains of Gram-negative bacteria, including Pseudomonas species, Escherichia coli, species of indole-positive and indole-negative Proteus, Providencia species, Klebsiella-Enterobacter-Serratia species, and Acinetobacter species. Clinical studies have shown Amikacin Sulfate Injection USP to be effective in bacterial septicemia ; in serious infections of the respiratory tract, bones and joints, central nervous system and skin and soft tissue; intra-abdominal infections ; and in burns and post-operative infections . Clinical studies have shown amikacin also to be effective in serious complicated and recurrent urinary tract infections due to these organisms. Aminoglycosides, including Amikacin Sulfate Injection USP are not indicated in uncomplicated initial episodes of urinary tract infections unless the causative organisms are not susceptible to antibiotics having less potential toxicity.Bacteriologic studies should be performed to identify causative organisms and their susceptibilities to amikacin. Amikacin may be considered as initial therapy in suspected Gram-negative infections and therapy may be instituted before obtaining the results of susceptibility testing. Clinical trials demonstrated that amikacin was effective in infections caused by gentamicin and/or tobramycin-resistant strains of Gram-negative organisms, particularly Proteus rettgeri, Providencia stuartii, Serratia marcescens, and Pseudomonas aeruginosa. The decision to continue therapy with the drug should be based on results of the susceptibility tests, the severity of the infection, the response of the patient and the important additional considerations contained in the WARNINGS box above. Amikacin has also been shown to be effective in staphylococcal infections and may be considered as initial therapy under certain conditions in the treatment of known or suspected staphylococcal disease such as, severe infections where the causative organism may be either a Gram-negative bacterium or a staphylococcus, infections due to susceptible strains of staphylococci in patients allergic to other antibiotics, and in mixed staphylococci/Gram-negative infections. In certain severe infections such as neonatal sepsis, concomitant therapy with a penicillin-type drug may be indicated because of the possibility of infections due to Gram-positive organisms such as streptococci or pneumococci. To reduce the development of drug-resistant bacteria and maintain the effectiveness of amikacin and other antibacterial drugs, amikacin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
ARIKAYCE (Amikacin)
WARNING: RISK OF INCREASED RESPIRATORY ADVERSE REACTIONS
ARIKAYCE has been associated with an increased risk of respiratory adverse reactions including, hypersensitivity pneumonitis, hemoptysis, bronchospasm, exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases
1INDICATIONS AND USAGE
LIMITED POPULATION: ARIKAYCE
This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by Month 6. Clinical benefit has not yet been established
2DOSAGE FORMS AND STRENGTHS
ARIKAYCE is supplied as a sterile, white, milky, aqueous, liposome suspension for oral inhalation in a unit-dose glass vial containing amikacin 590 mg/8.4 mL (equivalent to amikacin sulfate 788 mg/8.4 mL).
3CONTRAINDICATIONS
ARIKAYCE is contraindicated in patients with a known hypersensitivity to any aminoglycoside.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described in greater detail in other sections of labeling:
- Hypersensitivity pneumonitis
- Hemoptysis
- Bronchospasm
- Exacerbation of Underlying Pulmonary Disease
- Anaphylaxis and Hypersensitivity Reactions
- Ototoxicity
- Nephrotoxicity
- Neuromuscular Blockade
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following adverse reactions have been identified from postmarketing surveillance. Because these adverse reactions are reported voluntarily from a population of unknown size, precise estimates of frequency cannot be made and a causal relationship to drug exposure cannot be established.
Gastrointestinal disorders: dysphagia, glossitis, glossodynia, salivary hypersecretion, stomatitis, abdominal pain, abdominal discomfort, abdominal distension, abdominal pain upper
Immune system disorders: hypersensitivity, anaphylaxis
Respiratory, thoracic, and mediastinal disorders: nasal dryness, rhinorrhea, sneezing, nasal congestion
5OVERDOSAGE
Adverse reactions specifically associated with overdose of ARIKAYCE have not been identified. Acute toxicity should be treated with immediate withdrawal of ARIKAYCE, and baseline tests of renal function should be undertaken.
Hemodialysis may be helpful in removing amikacin from the body.
In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment. In the case of any overdosage, the possibility of drug interactions with alterations in drug disposition should be considered.
6DESCRIPTION
The active ingredient in ARIKAYCE (amikacin liposome inhalation suspension) is amikacin sulfate USP, an aminoglycoside antibacterial. Its chemical name is D-Streptamine,
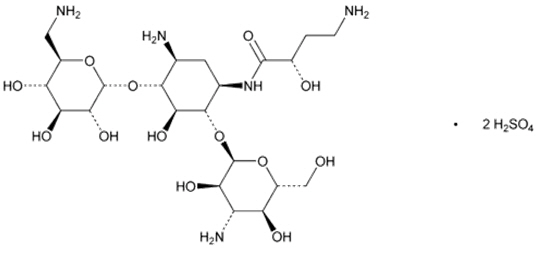
ARIKAYCE is a white milky suspension consisting of amikacin sulfate encapsulated in liposomes and is supplied in a unit-dose 10 mL clear glass vial containing amikacin 590 mg/8.4 mL (equivalent to amikacin sulfate 788 mg/8.4 mL) as a sterile aqueous liposomal suspension for oral inhalation. ARIKAYCE consists of amikacin sulfate encapsulated in liposomes at a targeted concentration of 70 mg amikacin/mL with the pH range of 6.1 to 7.1 and lipid to amikacin weight ratio in the range of 0.60 to 0.79. The inactive ingredients are cholesterol, dipalmitoylphosphatidylcholine (DPPC), sodium chloride, sodium hydroxide (for pH adjustment), and water for injection.
ARIKAYCE is administered only using a Lamira Nebulizer System
7CLINICAL STUDIES
Trial 1 (NCT#02344004) was an open-label, randomized (2:1), multi-center trial in patients with refractory
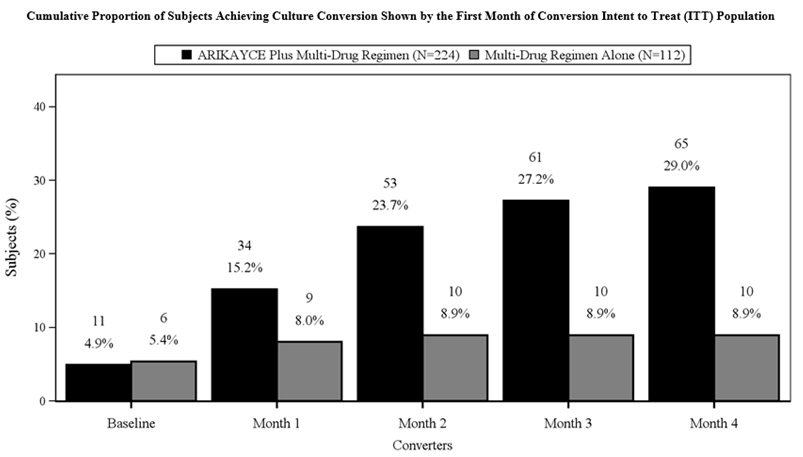
A total of 336 patients were randomized (ARIKAYCE plus background regimen, n=224; background regimen alone, n=112) (ITT population), with a mean age of 64.7 years and there was a higher percentage of females (69.3%) than males (30.7%) in the study. At the time of enrollment, of the 336 subjects in the ITT population, 302 (89.9%) were either on a guideline-based regimen for MAC or off guideline-based therapy for MAC for less than 3 months while 34 (10.1%) were off treatment for 3 to 12 months prior to enrollment. At screening, patients were stratified by smoking status (current smoker or not) and by whether patients were on treatment or off treatment for at least 3 months. Most patients at screening were not current smokers (89.3%) and had underlying bronchiectasis (62.5%). At baseline, 329 patients were on a multidrug background regimen that included a macrolide (93.3%), a rifamycin (86.3%), or ethambutol (81.4%). Overall, 55.6% of subjects were receiving a triple-drug background regimen consisting of a macrolide, a rifamycin and ethambutol.
The proportion of patients achieving culture conversion (3 consecutive monthly negative sputum cultures) by Month 6 was significantly (p<0.0001) greater for ARIKAYCE plus background regimen (65/224, 29.0%) compared to background regimen alone (10/112, 8.9%). Of those receiving ARIKAYCE plus background regimen, 18.3% (41/224) achieved culture conversion by Month 6 and sustained sputum culture conversion (defined as consecutive negative sputum cultures with no positive culture on solid media or no more than 2 consecutive positive cultures on liquid media following culture conversion) for up to 12 months of treatment after the first culture that defined culture conversion, compared to 2.7% (3/112) of patients receiving background regimen alone (p<0.0001). At 3 months after the completion of treatment, 16.1% (36/224) of patients who had received ARIKAYCE plus background regimen maintained durable culture conversion, compared to 0% of patients who had received background regimen alone (p<0.0001).
In Trial 1, 23/224 (10.3%) of patients had MAC isolates that developed MIC of > 64 mcg/mL while receiving treatment with ARIKAYCE. In the background regimen alone arm, 4/112 (3.6%) of patients had MAC isolates that developed amikacin MIC of > 64 mcg/mL.
Additional endpoints to assess the clinical benefit of ARIKAYCE, for example, change from baseline in six-minute walk test distance and the Saint George's Respiratory Questionnaire, did not demonstrate clinical benefit by Month 6.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Patient Instructions for Use).
9Instructions for Use
ARIKAYCE
For oral inhalation use
Before using your Lamira Nebulizer System, be sure you read and understand the detailed information in the full Instructions for Use that comes with the Lamira Nebulizer System. This will provide more complete information about how to put together (assemble), prepare, use, clean, and disinfect your Lamira Nebulizer System. If you do not understand any part of the instructions, contact
Cleaning and Disinfecting
Before first use, rinse, clean, and disinfect your Handset and Aerosol Head. Moving forward, rinse, clean, and disinfect the Handset including the Aerosol Head after every use.
When you receive your Handset and Aerosol Head, they will not be sterile. Cleaning and disinfecting your Handset and Aerosol Head is important to reduce the risk of infection, illness, and contamination.
Assembling Your Handset
Taking ARIKAYCE
Your ARIKAYCE should be at room temperature before use to make sure that your Lamira Nebulizer System operates properly.
Bring ARIKAYCE to room temperature by removing it from the refrigerator
Cleaning your Lamira Handset and Aerosol Head After Use
- Rinse, clean, and disinfect handset right away after each use to reduce infection, illness, and contamination.
- Disinfect the Handset and Aerosol Head after every use.
- See "
© 2018 Insmed Incorporated. All rights reserved. Insmed
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
10PRINCIPAL DISPLAY PANEL - 8.4 mL Vial Carton
1-833-LIGHT-00
Contains 28 sterile unit-dose vials
For oral inhalation only
ARIKAYCE
Limited Population*
insmed
Attention Patients:
*See the full prescribing information for ARIKAYCE
ARIKAYCE.COM
