Brand Name
Invokana
Generic Name
Canagliflozin
View Brand Information FDA approval date: March 29, 2013
Classification: Sodium-Glucose Cotransporter 2 Inhibitor
Form: Tablet
What is Invokana (Canagliflozin)?
INVOKANA is indicated: as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus., to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease ., to reduce the risk of end-stage kidney disease , doubling of serum creatinine, cardiovascular death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria greater than 300 mg/day. INVOKANA is a sodium-glucose co-transporter 2 inhibitor indicated:, as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus , to reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease , to reduce the risk of end-stage kidney disease, doubling of serum creatinine, cardiovascular death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria . Limitations of Use:, INVOKANA is not recommended in patients with type 1 diabetes mellitus. It may increase the risk of diabetic ketoacidosis in these patients , INVOKANA is not recommended for use to improve glycemic control in adults with type 2 diabetes mellitus with an eGFR less than 30 mL/min.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
INVOKANA (canagliflozin)
1INDICATIONS AND USAGE
INVOKANA (canagliflozin) is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, nonfatal myocardial infarction and nonfatal stroke) in adults with type 2 diabetes mellitus and established cardiovascular disease (CVD).
- to reduce the risk of end-stage kidney disease (ESKD), doubling of serum creatinine, cardiovascular (CV) death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria greater than 300 mg/day.
2DOSAGE FORMS AND STRENGTHS
- INVOKANA 100 mg tablets are yellow, capsule-shaped, tablets with "CFZ" on one side and "100" on the other side.
- INVOKANA 300 mg tablets are white, capsule-shaped, tablets with "CFZ" on one side and "300" on the other side.
3CONTRAINDICATIONS
INVOKANA is contraindicated in patients with a serious hypersensitivity reaction to INVOKANA, such as anaphylaxis or angioedema
4ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes and Other Ketoacidosis
- Lower Limb Amputation
- Volume Depletion
- Urosepsis and Pyelonephritis
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
- Necrotizing Fasciitis of the Perineum (Fournier's gangrene)
- Genital Mycotic Infections
- Hypersensitivity Reactions
- Bone Fracture
4.1Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
4.2Postmarketing Experience
Additional adverse reactions have been identified during post-approval use of INVOKANA. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Ketoacidosis
Acute Kidney Injury
Anaphylaxis, Angioedema
Urosepsis and Pyelonephritis
Necrotizing Fasciitis of the Perineum (Fournier's gangrene)
5OVERDOSAGE
In the event of an overdose, contact the Poison Control Center. It is also reasonable to employ the usual supportive measures, e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive treatment as dictated by the patient's clinical status. Canagliflozin was negligibly removed during a 4-hour hemodialysis session. Canagliflozin is not expected to be dialyzable by peritoneal dialysis.
6DESCRIPTION
INVOKANA
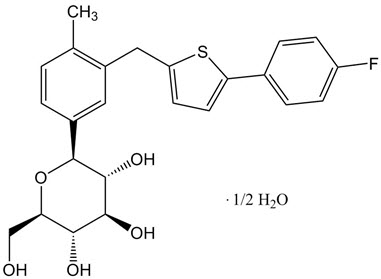
Canagliflozin is practically insoluble in aqueous media from pH 1.1 to 12.9.
INVOKANA is supplied as film-coated tablets for oral administration, containing 102 and 306 mg of canagliflozin in each tablet strength, corresponding to 100 mg and 300 mg of canagliflozin (anhydrous), respectively.
Inactive ingredients of the core tablet are croscarmellose sodium, hydroxypropyl cellulose, lactose anhydrous, magnesium stearate, and microcrystalline cellulose. The magnesium stearate is vegetable-sourced. The tablets are finished with a commercially available film-coating consisting of the following excipients: polyvinyl alcohol (partially hydrolyzed), titanium dioxide, macrogol/PEG, talc, and iron oxide yellow, E172 (100 mg tablet only).
7HOW SUPPLIED/STORAGE AND HANDLING
Product: 50090-5029
NDC: 50090-5029-0 90 TABLET, FILM COATED in a BOTTLE
8PATIENT COUNSELING INFORMATION
See .
9canagliflozin
