Brand Name
Zykadia
Generic Name
Ceritinib
View Brand Information FDA approval date: March 18, 2019
Classification: Kinase Inhibitor
Form: Tablet
What is Zykadia (Ceritinib)?
ZYKADIA ® is indicated for the treatment of adult patients with metastatic non-small cell lung cancer whose tumors are anaplastic lymphoma kinase -positive as detected by an FDA-approved test [see Dosage and Administration.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Study Using Molecular Guided Therapy With Induction Chemotherapy Followed by a Randomized Controlled Trial of Standard Immunotherapy With or Without DFMO Followed by DFMO Maintenance for Subjects With Newly Diagnosed High-Risk Neuroblastoma
Summary: A prospective open label, multicenter study to evaluate the feasibility and acute toxicity of using molecularly guided therapy in combination with standard therapy followed by a Randomized Controlled Trial of standard immunotherapy with or without DFMO followed by DFMO maintenance for Subjects with Newly Diagnosed High-Risk Neuroblastoma.
Population Pharmacokinetics, Effectiveness and Safety of Antineoplastic Drugs in Elderly Patients
Summary: The purpose is to study the population pharmacokinetics, effectiveness and safety of antineoplastic drugs (Busulfan, Paclitaxel, Afatinib, Ceritinib, Crizotinib, Imatinib, Lapatinib, etc) in elderly patients and recommend optimized dosage regimens.
Related Latest Advances
Brand Information
ZYKADIA (ceritinib)
1INDICATIONS AND USAGE
ZYKADIA
2DOSAGE FORMS AND STRENGTHS
Capsules: 150 mg hard gelatin capsule with opaque blue cap and opaque white body containing a white to off-white powder. The opaque blue cap is marked in black ink with “LDK 150MG” and the opaque white body is marked in black ink with “NVR”.
Tablets: 150 mg film-coated tablet, light blue, round, biconvex with beveled edges, without score, debossed with “NVR” on one side and “ZY1” on the other side.
3CONTRAINDICATIONS
None.
4ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Gastrointestinal Adverse Reactions
- Hepatotoxicity
- Interstitial Lung Disease/Pneumonitis
- QT Interval Prolongation
- Hyperglycemia
- Bradycardia
- Pancreatitis
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the Warnings and Precautions section reflect exposure to ZYKADIA 750 mg once daily under fasted conditions in 925 patients with ALK-positive NSCLC across seven clinical studies, including ASCEND-4 and ASCEND-1, described below, a randomized active-controlled study, two single arm studies, and two dose-escalation studies. The majority of patients enrolled in these studies had received prior treatment with chemotherapy and/or crizotinib for NSCLC. Among these 925 patients, the most common adverse reactions (≥ 25% incidence) were diarrhea, nausea, vomiting, fatigue, abdominal pain, decreased appetite, and weight loss. Approximately 45% of patients initiating treatment with ZYKADIA 750 mg under fasted conditions had an adverse reaction that required at least one dose reduction and 66% of patients had an adverse reaction that required at least one dose interruption. The median time to first dose reduction due to any reason was 7 weeks.
Dose Optimization Study: Dosing Regimen of 450 mg Daily With Food
In ASCEND-8, a dose optimization study, ZYKADIA 450 mg daily with food (N = 108) was compared to 750 mg daily under fasted conditions (N = 110) in both previously treated and untreated patients with ALK-positive NSCLC. The overall safety profile of ZYKADIA 450 mg with food was consistent with ZYKADIA 750 mg fasted, except for a reduction in gastrointestinal adverse reactions, while achieving comparable steady-state exposure
In patients treated with ZYKADIA 450 mg with food, 24% of patients had an adverse reaction that required at least one dose reduction and 56% of patients had an adverse reaction that required at least one dose interruption. The median time to first dose reduction due to any reason was 8 weeks.
Previously Untreated ALK-Positive Metastatic NSCLC
The safety of ZYKADIA was evaluated in ASCEND-4, an open-label, randomized, active-controlled multicenter study of 376 previously untreated ALK-positive NSCLC patients
The demographic characteristics of the study population were 57% female, median age 54 years (range, 22 to 81 years), 22% age 65 years or older, 54% white, 42% Asian, 2% black, and 2% other races. Patients were enrolled in Europe (53%), Asia Pacific (42%), and South America (5%) regions. The majority of patients had adenocarcinoma (97%), never smoked (61%), and 32% had brain metastases at screening.
The following fatal adverse reactions occurred in 4 patients treated with ZYKADIA: Myocardial infarction, respiratory tract infection, pneumonitis, and unknown cause.
Serious adverse reactions were reported in 38% of patients treated with ZYKADIA. The most frequent serious adverse reactions were pneumonia (4%), pleural effusion (4%), vomiting (4%), nausea (3%), dyspnea (3%), hyperglycemia (3%), AST increased (2%), lung infection (2%), and pericardial effusion (2%).
Among patients treated with ZYKADIA, dose interruptions due to adverse reactions occurred in 77%, dose reductions were required in 66%, and adverse reactions that led to discontinuation of therapy occurred in 12% of patients. The most frequent adverse reactions, reported in at least 10% of patients treated with ZYKADIA, that led to dose interruptions or reductions were: Increased ALT (48%), increased AST (34%), vomiting (15%), increased blood creatinine (14%), increased gamma-glutamyl transpeptidase (GGT) (13%), diarrhea (13%), and nausea (13%). The most frequent adverse reactions that led to discontinuation of ZYKADIA in 1% or more of patients in ASCEND-4 were increased blood creatinine (2.1%), increased amylase (1.1%), and increased lipase (1.1%).
Tables 3 and 4 summarize adverse reactions and laboratory abnormalities, respectively, in ASCEND-4.
Additional clinically significant adverse reactions occurring in 2% or more of patients treated with ZYKADIA 750 mg under fasted conditions included: vision disorder (4% comprised of vision impairment, blurred vision, photopsia, accommodation disorder, presbyopia, reduced visual acuity, or vitreous floaters), bradycardia (4%), ILD/pneumonitis (2%), hepatotoxicity (2%), and renal failure (2%). In addition, the adverse reaction of photosensitivity was reported in 1.1% of patients.
Previously Treated ALK-Positive Metastatic NSCLC
The safety of ZYKADIA was evaluated in ASCEND-1, a multicenter, single-arm, open-label clinical study of 255 ALK-positive patients (246 patients with NSCLC and 9 patients with other cancers who received ZYKADIA at a dose of 750 mg daily under fasted conditions)
The study population characteristics were: Median age 53 years, age less than 65 (84%), female (53%), white (63%), Asian (34%), NSCLC adenocarcinoma histology (90%), never or former smoker (97%), Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 or 1 (89%), brain metastases (49%), and number of prior therapies 2 or more (67%).
Fatal adverse reactions in patients treated with ZYKADIA occurred in 5% of patients, consisting of: Pneumonia (4 patients), respiratory failure, ILD/pneumonitis, pneumothorax, gastric hemorrhage, general physical health deterioration, pulmonary tuberculosis, cardiac tamponade, and sepsis (1 patient each).
Serious adverse reactions reported in 2% or more of patients in ASCEND-1 were convulsion, pneumonia, ILD/pneumonitis, dyspnea, dehydration, hyperglycemia, and nausea.
Dose reductions due to adverse reactions occurred in 59% of patients treated with ZYKADIA. The most frequent adverse reactions, reported in at least 10% of patients, that led to dose reductions or interruptions were: Increased ALT (29%), nausea (20%), increased AST (16%), diarrhea (16%), and vomiting (16%). Discontinuation of therapy due to adverse reactions occurred in 10% of patients treated with ZYKADIA. The most frequent adverse reactions that led to discontinuation in 1% or more of patients in ASCEND-1 were pneumonia, ILD/pneumonitis, and decreased appetite.
Tables 5 and 6 summarize adverse reactions and laboratory abnormalities, respectively, in ASCEND-1.
Additional clinically significant adverse reactions occurring in 2% or more of patients treated with ZYKADIA 750 mg under fasted conditions included neuropathy (17% comprised of paresthesia, muscular weakness, gait disturbance, peripheral neuropathy, hypoesthesia, peripheral sensory neuropathy, dysesthesia, neuralgia, peripheral motor neuropathy, hypotonia, or polyneuropathy), vision disorder (9% comprised of vision impairment, blurred vision, photopsia, accommodation disorder, presbyopia, or reduced visual acuity), prolonged QT interval (4%), and bradycardia (3%). In addition, the adverse reaction of photosensitivity was reported in 1.2% of patients.
5DESCRIPTION
Ceritinib is a kinase inhibitor for oral administration. The molecular formula for ceritinib is C
The chemical structure of ceritinib is shown below:
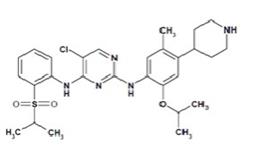
Ceritinib is a white to almost white or light yellow powder.
ZYKADIA is supplied as printed hard-gelatin capsules containing 150 mg of ceritinib and the following inactive ingredients: colloidal silicon dioxide, hard gelatin capsule shells, low substituted hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The capsule shell is composed of FD&C Blue # 2, gelatin, and titanium dioxide.
ZYKADIA is supplied as film-coated tablets containing 150 mg of ceritinib and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, low-substituted hydroxypropyl cellulose, magnesium stearate, microcrystalline cellulose and povidone. The tablet coating contains FD&C Blue # 2 aluminum lake, hypromellose, polyethylene glycol 4000, talc, and titanium dioxide.
6HOW SUPPLIED/STORAGE AND HANDLING
ZYKADIA 150 mg capsules
Hard gelatin capsule with opaque blue cap and opaque white body; opaque blue cap marked in black ink with “LDK 150MG”, opaque white body marked in black ink with “NVR”. Available in:
Bottles of 70 capsules…………………………………………………………………………………….NDC 0078-0640-70
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
ZYKADIA 150 mg tablets
Film-coated tablet, light blue, round, biconvex with beveled edges, without score, debossed with “NVR” on one side and “ZY1” on the other side. Available in:
Bottles of 84 tablets…………………………………………………………………………………NDC 0078-0694-84
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information)
Gastrointestinal Adverse Reactions
Inform patients that diarrhea, nausea, vomiting, and abdominal pain are the most commonly reported gastrointestinal adverse reactions. Inform patients of supportive care options, such as antiemetic and anti-diarrheal medications. Advise patients to contact their healthcare provider for severe or intolerable gastrointestinal symptoms. Inform patients that if vomiting occurs during the course of treatment, they should not take an additional dose, but should continue with the next scheduled dose of ZYKADIA
Hepatotoxicity
Inform patients of the signs and symptoms of hepatotoxicity. Advise patients to contact their healthcare provider immediately for signs or symptoms of hepatotoxicity
Interstitial Lung Disease/Pneumonitis
Inform patients of the risks of severe or fatal ILD/pneumonitis. Advise patients to contact their healthcare provider immediately to report new or worsening respiratory symptoms
Arrhythmias
Inform patients of the risks of QTc interval prolongation and bradycardia. Advise patients to contact their healthcare provider immediately to report new chest pain or discomfort, changes in heartbeat, palpitations, dizziness, lightheadedness, fainting, and changes in or new use of heart or blood pressure medications
Hyperglycemia
Inform patients of the signs and symptoms of hyperglycemia. Advise patients to contact their healthcare provider immediately for signs or symptoms of hyperglycemia
Pancreatitis
Inform patients of the signs and symptoms of pancreatitis and the need to monitor lipase and amylase levels prior to the start of treatment and periodically thereafter as clinically indicated
Photosensitivity
Inform patients of the signs and symptoms of photosensitivity. Advise patients to avoid prolonged sun exposure and to use sunscreen or protective clothing during treatment with ZYKADIA
Embryo-Fetal Toxicity
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy
- Advise females of reproductive potential to use effective contraception during treatment with ZYKADIA and for 6 months following completion of therapy
- Advise males with female partners of reproductive potential to use condoms during treatment with ZYKADIA and for 3 months following completion of therapy
Lactation
Advise women not to breastfeed during treatment with ZYKADIA and for 2 weeks following completion of therapy
Drug Interactions
Inform patients not to consume grapefruit and grapefruit juice during treatment with ZYKADIA
Dosing Instructions
Advise patients to take ZYKADIA with food
Distributed by:
© Novartis
T2021-132
8PRINCIPAL DISPLAY PANEL
NDC 0078-0694-84
Zykadia
(ceritinib) tablets
150 mg
84 Tablets
Rx only
NOVARTIS