Brand Name
Aciphex
Generic Name
Rabeprazole
View Brand Information FDA approval date: November 08, 2013
Classification: Proton Pump Inhibitor
Form: Tablet
What is Aciphex (Rabeprazole)?
Rabeprazole Sodium Delayed-Release Tablets is a proton pump inhibitor indicated in adults for: Healing of Erosive or Ulcerative Gastroesophageal Reflux Disease
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Aciphex (rabeprazole sodium)
1DOSAGE AND ADMINISTRATION
Table 1 shows the recommended dosage of ACIPHEX delayed-release tablets in adults and adolescent patients 12 years of age and older. The use of ACIPHEX delayed-release tablets is not recommended for use in pediatric patients 1 year to less than 12 years of age because the lowest available tablet strength (20 mg) exceeds the recommended dose for these patients. Use another rabeprazole formulation for pediatric patients 1 year to less than 12 years of age.
2DOSAGE FORMS AND STRENGTHS
ACIPHEX delayed-release tablets are provided in one strength, 20 mg. The tablets are round, light yellow, enteric coated, biconvex tablets. "ACIPHEX 20" is imprinted in red on one side of the tablet.
3CONTRAINDICATIONS
- ACIPHEX is contraindicated in patients with known hypersensitivity to rabeprazole, substituted benzimidazoles, or to any component of the formulation. Hypersensitivity reactions may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, acute tubulointerstitial nephritis, and urticaria
- PPIs, including ACIPHEX, are contraindicated with rilpivirine-containing products
- For information about contraindications of antibacterial agents (clarithromycin and amoxicillin) indicated in combination with ACIPHEX delayed-release tablets, refer to the
4ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in labeling:
- Acute Tubulointerstitial Nephritis
- Clostridium difficile-Associated Diarrhea [see
- Bone Fracture
- Cutaneous and Systemic Lupus Erythematosus
- Cyanocobalamin (Vitamin B-12) Deficiency
- Hypomagnesemia and Mineral Metabolism
- Fundic Gland Polyps
4.1Clinical Studies Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
4.2Postmarketing Experience
The following adverse reactions have been identified during post approval use of rabeprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Blood and Lymphatic System Disorders: agranulocytosis, hemolytic anemia, leukopenia, pancytopenia, thrombocytopenia
Ear and Labyrinth Disorders: vertigo
Eye Disorders: blurred vision
Gastrointestinal Disorders: fundic gland polyps
General Disorders and Administration Site Conditions: sudden death
Hepatobiliary Disorders: jaundice
Immune System Disorders: anaphylaxis, angioedema, systemic lupus erythematosus, Stevens-Johnson syndrome, toxic epidermal necrolysis (some fatal), DRESS, AGEP
Infections and Infestations: Clostridium difficile-associated diarrhea
Investigations: Increases in prothrombin time/INR (in patients treated with concomitant warfarin), TSH elevations
Metabolism and Nutrition Disorders: hyperammonemia, hypomagnesemia, hypocalcemia, hypokalemia [Warnings and Precautions 5.8], hyponatremia
Musculoskeletal System Disorders: bone fracture, rhabdomyolysis
Nervous System Disorders: coma
Psychiatric Disorders: delirium, disorientation
Renal and Genitourinary Disorders: interstitial nephritis, erectile dysfunction
Respiratory, Thoracic and Mediastinal Disorders: interstitial pneumonia
Skin and Subcutaneous Tissue Disorders: severe dermatologic reactions including bullous and other drug eruptions of the skin, cutaneous lupus erythematosus, erythema multiforme
5DRUG INTERACTIONS
Table 2 includes drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with ACIPHEX delayed-release tablets and instructions for preventing or managing them.
Consult the labeling of concomitantly used drugs to obtain further information about interactions with PPIs.
6OVERDOSAGE
Seven reports of accidental overdosage with rabeprazole have been received. The maximum reported overdose was 80 mg. There were no clinical signs or symptoms associated with any reported overdose. Patients with Zollinger-Ellison syndrome have been treated with up to 120 mg rabeprazole once daily. No specific antidote for rabeprazole is known. Rabeprazole is extensively protein bound and is not readily dialyzable.
In the event of overdosage, treatment should be symptomatic and supportive.
If over-exposure occurs, call your Poison Control Center at 1-800-222-1222 for current information on the management of poisoning or overdosage.
7DESCRIPTION
The active ingredient in ACIPHEX delayed-release tablets is rabeprazole sodium, which is a proton pump inhibitor. It is a substituted benzimidazole known chemically as 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1
Figure 1
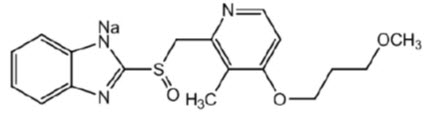
ACIPHEX is available for oral administration as delayed-release, enteric-coated tablets containing 20 mg of rabeprazole sodium.
Inactive ingredients of the 20 mg tablet are carnauba wax, crospovidone, diacetylated monoglycerides, ethylcellulose, hydroxypropyl cellulose, hypromellose phthalate, magnesium stearate, mannitol, sodium hydroxide, sodium stearyl fumarate, talc, and titanium dioxide. Iron oxide yellow is the coloring agent for the tablet coating. Iron oxide red is the ink pigment.
8REFERENCES
1. Clinical and Laboratory Standards Institute (CLSI).
9HOW SUPPLIED/STORAGE AND HANDLING
ACIPHEX 20 mg is supplied as delayed-release light yellow enteric-coated tablets. The name and strength, in mg, (ACIPHEX 20) is imprinted on one side.
- Bottles of 30 (NDC 80725-243-30)
10PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
11PRINCIPAL DISPLAY PANEL - 30 Tablet Bottle Label
NDC 80725-243-30
Rx only
AcipHex
rabeprazole sodium
delayed-release
20 mg
Waylis
30 tablets
