Brand Name
Eucrisa
Generic Name
Crisaborole
View Brand Information FDA approval date: January 30, 2017
Classification: Phosphodiesterase 4 Inhibitor
Form: Ointment
What is Eucrisa (Crisaborole)?
EUCRISA is indicated for topical treatment of mild to moderate atopic dermatitis in adult and pediatric patients 3 months of age and older. EUCRISA is a phosphodiesterase 4 inhibitor indicated for topical treatment of mild to moderate atopic dermatitis in adult and pediatric patients 3 months of age and older.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Efficacy and Safety of Crisaborole Ointment, a Phosphodiesterase 4 (PDE4) Inhibitor, for the Topical Treatment of Cetuximab-Related Skin Toxicity Among Metastatic Colorectal Cancer Patients:A Prospective, Single-arm, Phase II Clinical Trial
Summary: This is a prospective, single-arm, phase II clinical trial that will enroll metastatic colorectal cancer patients with Cetuximab-Related Skin Toxicity, who will receive crisaborole ointment twice daily.
Related Latest Advances
Brand Information
Eucrisa (crisaborole)
1INDICATIONS AND USAGE
EUCRISA is indicated for topical treatment of mild to moderate atopic dermatitis in adult and pediatric patients 3 months of age and older.
2DOSAGE AND ADMINISTRATION
Apply a thin layer of EUCRISA twice daily to affected areas. Once clinical effect is achieved, consider reducing application to once daily
EUCRISA is for topical use only and not for ophthalmic, oral, or intravaginal use.
3DOSAGE FORMS AND STRENGTHS
Ointment: 20 mg of crisaborole per gram (2%) of white to off-white ointment.
4CONTRAINDICATIONS
EUCRISA is contraindicated in patients with known hypersensitivity to crisaborole or any component of the formulation.
5DESCRIPTION
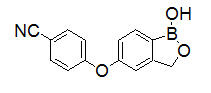
EUCRISA contains 2% crisaborole (w/w) in a petrolatum-based, white to off-white ointment and is for topical use. The active ingredient, crisaborole, is a phosphodiesterase-4 (PDE-4) inhibitor.
Crisaborole is described chemically as 5-(4-cyanophenoxy)-1,3-dihydro-1-hydroxy-[2,1]-benzoxaborole. The empirical formula is C
The structural formula is represented below:
Crisaborole drug substance is freely soluble in common organic solvents such as isopropyl alcohol and propylene glycol, and insoluble in water.
Each gram of EUCRISA contains 20 mg of crisaborole in an ointment containing white petrolatum, propylene glycol, mono- and di-glycerides, paraffin, butylated hydroxytoluene, and edetate calcium disodium.
6CLINICAL STUDIES
Two multicenter, randomized, double-blind, parallel-group, vehicle-controlled trials (Trials 1 and 2) treated a total of 1522 subjects 2 to 79 years of age (86.3% of subjects were 2 to 17 years of age) with a 5% to 95% treatable BSA. At baseline, 38.5% of the subjects had an Investigator's Static Global Assessment [ISGA] of mild (2), and 61.5% had an ISGA of moderate (3), in the overall assessment of atopic dermatitis (erythema, induration/papulation, and oozing/crusting) on a severity scale of 0 to 4.
In both trials, subjects were randomized 2:1 to receive EUCRISA or vehicle applied twice daily for 28 days. The primary efficacy endpoint was the proportion of subjects at Day 29 who achieved success, defined as an ISGA grade of clear (0) or almost clear (1) with a 2-grade or greater improvement from baseline, comparing EUCRISA-treated subjects to vehicle-treated subjects.
Efficacy results from the two trials are summarized in Table 2.
The success rates over time are presented in Figure 1.
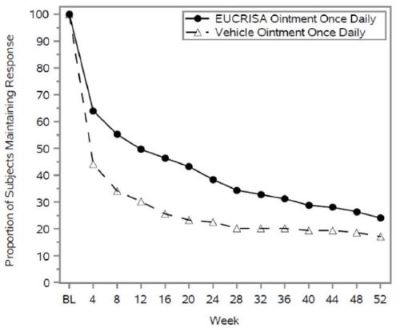
One randomized, double-blind, vehicle-controlled trial (Trial 3) assessed the efficacy and safety of EUCRISA once daily over 52 weeks in pediatric (3 months to less than 18 years of age) and adult subjects with mild to moderate atopic dermatitis, who achieved success on EUCRISA twice daily during open-label treatment of up to 8 weeks.
A total of 497 subjects 3 months of age and older with a 2% to 90% treatable BSA, entered into an open-label period to receive EUCRISA twice daily for up to 8 weeks. At baseline, 327 (66%) of subjects were 3 months to less than 18 years of age, 66% of the subjects had an ISGA of moderate (3), and 34% had an ISGA of mild (2), in the overall assessment of atopic dermatitis (erythema, induration/papulation, and oozing/crusting) on a severity scale of 0 to 4.
Of the 497, a total of 254 subjects 3 months of age and older, who achieved both ISGA success (score of clear [0] or almost clear [1] with a ≥2 grade improvement from baseline) and EASI50 response (at least 50% improvement from baseline in EASI scores) were randomized 1:1 into a double-blind period to receive EUCRISA once daily or vehicle for 52 weeks or until they developed a flare. At the beginning of the double-blind period, 59% of the subjects had an ISGA of almost clear (1) and 41% had an ISGA of clear (0).
Figure 2 presents the percentage of subjects maintaining an ISGA of clear or almost clear through Week 52.
Figure 2: Percentage of Subjects Maintaining ISGA of Clear or Almost Clear Through Week 52
7PATIENT COUNSELING INFORMATION
Advise the patient or caregivers to read the FDA-approved patient labeling (Patient Information).
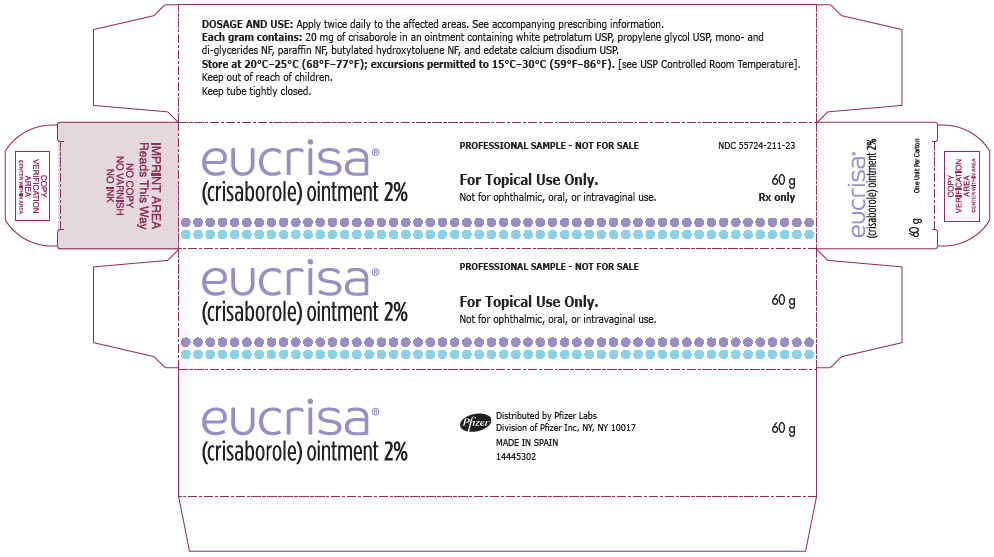
8PRINCIPAL DISPLAY PANEL - 60 g Tube Label
eucrisa
NDC 55724-211-21
For Topical Use Only.
Not for ophthalmic, oral, or intravaginal use.

9PRINCIPAL DISPLAY PANEL - 60 g Tube Carton
eucrisa
For Topical Use Only.
NDC 55724-211-21
60 g
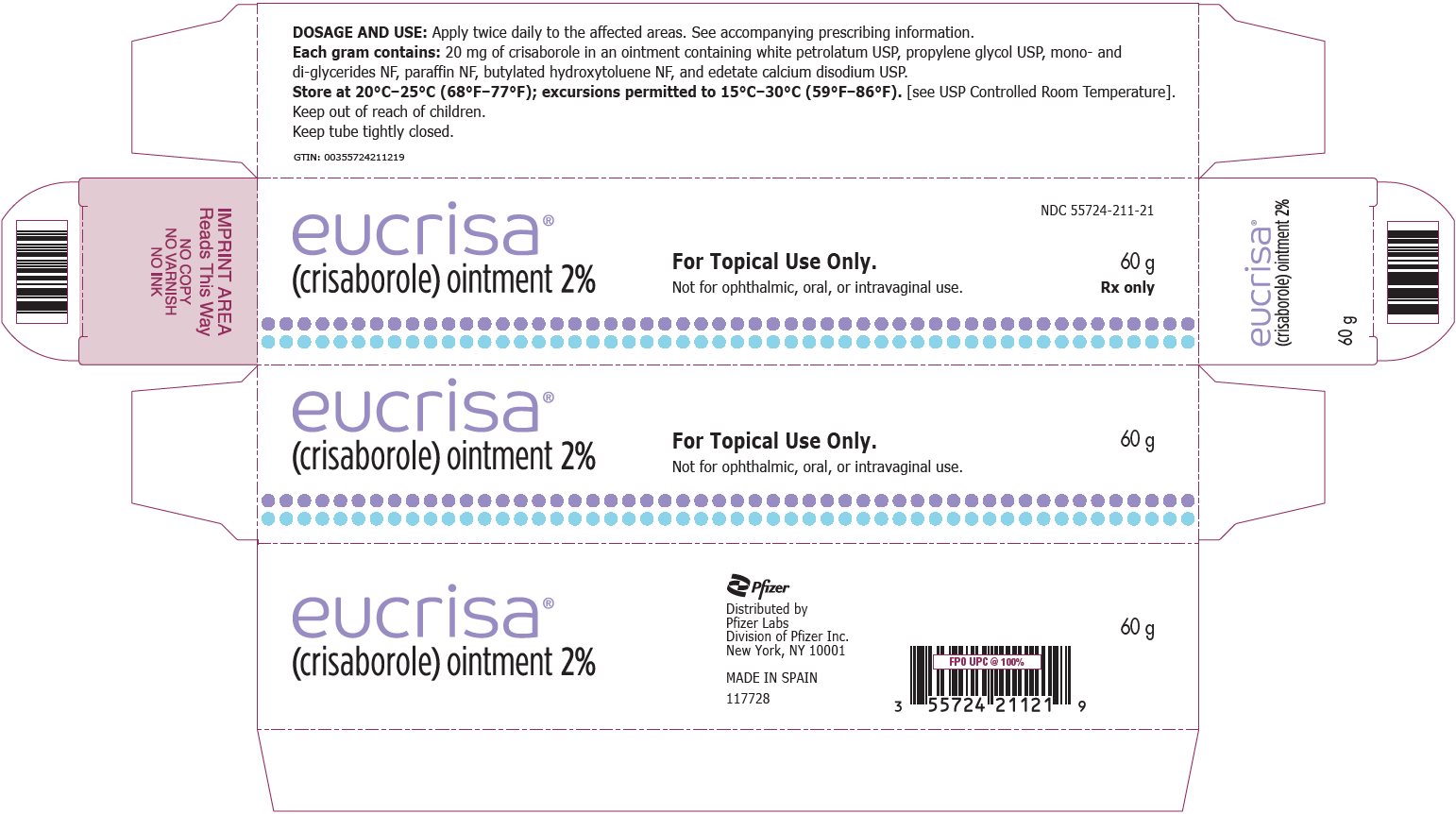
10PRINCIPAL DISPLAY PANEL - 60 g Tube Label - Sample
eucrisa
PROFESSIONAL SAMPLE - NOT FOR SALE
60 g
For Topical Use Only.
Not for ophthalmic, oral, or intravaginal use.

11PRINCIPAL DISPLAY PANEL - 60 g Tube Carton - Sample
eucrisa
PROFESSIONAL SAMPLE - NOT FOR SALE
For Topical Use Only.
NDC 55724-211-23
60 g