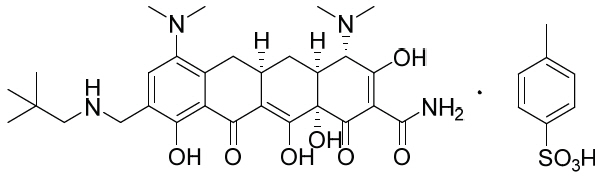
Nuzyra
What is Nuzyra (Omadacycline)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The study design is a randomized, open-label, clinical trial of omadacycline vs Standard of Care (SOC) antibiotics for bone and join infection (BJI) treatment. Study participants will have their BJI regimen chosen by their treating physicians, (typically Infectious Diseases for hardware and prosthetic joint infections, or multidisciplinary Limb Salvage team for diabetic foot infections) prior to e...
Summary: This will be a prospective, open-label, two-center study to assess the safety of omadacycline use in the treatment of hospitalized subjects with moderate to severe DFI with or without Acute osteomyelitis (AOM) who are at a high risk for development of CDI, AKI, and/or resistant pathogens compared to retrospective controls. Prospective enrollment will be continued until the sample size is achieved ...
Summary: The purpose of this study is to evaluate the pharmacokinetics of a single dose of intravenous or oral omadacycline in children and adolescents with suspected or confirmed bacterial infections.
Related Latest Advances
Brand Information
- Mortality Imbalance in Patients with Community-Acquired Bacterial Pneumonia
- Tooth Development and Enamel Hypoplasia
- Inhibition of Bone Growth
- Hypersensitivity Reactions
- Tetracycline Class Effects