Khapzory
What is Khapzory (Levoleucovorin)?
For people undergoing cancer treatment, the journey can be physically and emotionally demanding. Chemotherapy, while vital for fighting cancer cells, can also harm healthy tissues and cause challenging side effects. Khapzory (levoleucovorin) is a medication designed to protect healthy cells and enhance the effectiveness of certain chemotherapy drugs, helping patients tolerate treatment better and improve outcomes.
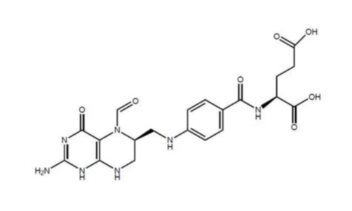
Khapzory belongs to a group of medications known as chemoprotective agents or folate analogs. It is the active form of leucovorin, a drug that supports the body’s use of folic acid, an essential vitamin for cell repair and DNA synthesis. Approved by the U.S. Food and Drug Administration (FDA), Khapzory is used in combination with specific cancer therapies or as a rescue medication after high-dose chemotherapy. It plays a critical role in balancing efficacy with safety, allowing patients to benefit from powerful treatments while reducing harmful side effects.
What does Khapzory do?
Khapzory is used primarily in cancer care and certain medication overdose situations. Its main purposes include:
- Enhancing chemotherapy effectiveness: Khapzory is often combined with fluorouracil (5-FU) to treat cancers such as colorectal cancer. In this setting, it strengthens the cancer-fighting effect of fluorouracil, helping the drug work more efficiently against tumor cells.[Text Wrapping Break]
- Reducing chemotherapy toxicity: After high doses of methotrexate, which can damage healthy cells, Khapzory acts as a rescue agent to protect normal tissues. This is known as “leucovorin rescue,” and it allows higher doses of methotrexate to be used safely in treating cancers like leukemia or osteosarcoma.[Text Wrapping Break]
- Treating folate deficiency or overdose: In some cases, Khapzory may also be used to reverse the effects of folate-blocking medications or to help manage toxic reactions caused by certain drugs.
Patients receiving Khapzory as part of chemotherapy typically experience better tolerance to treatment and improved recovery of normal cell function. Clinical studies have shown that using levoleucovorin instead of racemic leucovorin provides a more efficient active form of the drug, meaning the body can use it immediately without needing to convert it (NIH, 2024).
How does Khapzory work?
Khapzory works by supplying the body with levoleucovorin, the biologically active form of folinic acid. To understand how it works, it helps to know that folic acid is essential for DNA production and cell repair, both of which are vital during cancer treatment.
Some chemotherapy drugs, like methotrexate, block the body’s ability to use folic acid, stopping cancer cells from dividing. While this approach kills cancer cells, it can also harm normal cells that need folic acid to survive. Khapzory “rescues” these healthy cells by bypassing the blocked pathway and restoring the folate balance, allowing them to recover.
In other cases, such as with fluorouracil therapy, Khapzory has the opposite role: it boosts the anticancer effect. When combined with fluorouracil, levoleucovorin enhances the drug’s ability to interfere with DNA production in cancer cells, making the chemotherapy more potent.
Clinically, this dual role protecting normal cells while strengthening chemotherapy makes Khapzory a critical supportive treatment in oncology care.
Khapzory side effects
Khapzory is generally well-tolerated, but like all medications, it can cause side effects. The type and severity often depend on the patient’s overall health and the other drugs being used alongside it.
Common side effects may include:
- Nausea or vomiting[Text Wrapping Break]
- Diarrhea or stomach upset[Text Wrapping Break]
- Fatigue or weakness[Text Wrapping Break]
- Mouth sores[Text Wrapping Break]
- Mild redness or irritation at the injection site
Serious side effects (less common):
- Allergic reactions such as rash, itching, or swelling of the face or throat[Text Wrapping Break]
- Trouble breathing or dizziness[Text Wrapping Break]
- Seizures (rare, but possible with very high doses or certain medical conditions)
Khapzory with fluorouracil can worsen chemotherapy side effects like mouth sores, diarrhea, or low white blood cell counts. Use with caution in dehydrated patients, those with kidney problems, or folinic acid hypersensitivity.
Seek immediate medical attention for allergic reactions or severe diarrhea. Most patients tolerate Khapzory well with regular supervision to manage side effects.
Khapzory dosage
Khapzory is an IV injection or infusion given by a healthcare professional. Dosage and schedule vary based on cancer type, chemotherapy, body size, and kidney function. With methotrexate, it’s started hours after chemo and continued until methotrexate clears. With fluorouracil, it’s given the same day to enhance effect.
Doctors monitor blood counts, kidney function, and methotrexate levels (if applicable) for treatment safety. Patients should stay hydrated and report unusual symptoms. Older adults or those with kidney impairment may need dosage adjustments due to slower drug clearance.
Does Khapzory have a generic version?
Yes. Levoleucovorin, the active ingredient in Khapzory, is available in the United States as both a brand-name and generic injectable formulation. FDA-approved generic versions are considered equally safe and effective as Khapzory because they contain the same active ingredient and are held to identical manufacturing and quality standards.
Khapzory, like other levoleucovorin formulations such as Fusilev, is interchangeable. The choice depends on insurance, availability, and treatment setting, but efficacy is maintained when properly administered.
Conclusion
Khapzory (levoleucovorin) is a vital part of modern cancer therapy, serving both as a rescue medication and a chemotherapy enhancer. By protecting healthy cells from methotrexate toxicity and boosting the action of fluorouracil, it allows oncologists to use powerful treatments more safely and effectively.
Side effects are typically mild and manageable with professional oversight, ensuring treatment effectively targets cancer while protecting healthy tissue. Understanding Khapzory’s role in the treatment plan empowers patients and caregivers. Prescribed and monitored by experts, Khapzory aids the body’s resilience, helping patients move forward with strength.
References
- U.S. Food and Drug Administration (FDA). (2024). Khapzory (levoleucovorin) injection prescribing information. Retrieved from https://www.accessdata.fda.gov[Text Wrapping Break]
- MedlinePlus. (2024). Levoleucovorin injection: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov[Text Wrapping Break]
- Mayo Clinic. (2024). Levoleucovorin (injection route) description and precautions. Retrieved from https://www.mayoclinic.org[Text Wrapping Break]
- National Institutes of Health (NIH). (2024). Folinic acid rescue and chemotherapy modulation overview. Retrieved from https://www.nih.gov[Text Wrapping Break]
Top Global Experts
Related Clinical Trials
Summary: This phase II trial tests the addition of venetoclax and/or blinatumomab to usual chemotherapy for treating infants with newly diagnosed acute lymphoblastic leukemia (ALL) with a KMT2A gene rearrangement (KMT2A-rearranged \[R\]) or without a KMT2A gene rearrangement (KMT2A-germline \[G\]). Venetoclax is in a class of medications called B-cell lymphoma-2 (Bcl-2) inhibitors. It may stop the growth o...
Summary: The purpose of this study is to compare how long the participants are disease-free (progression-free survival) when treated with amivantamab and chemotherapy with 5-fluorouracil, leucovorin calcium (folinic acid) or levoleucovorin, oxaliplatin (mFOLFOX6) or 5-fluorouracil, leucovorin calcium (folinic acid) or levoleucovorin, and irinotecan hydrochloride (FOLFIRI) versus cetuximab and mFOLFOX6 or F...
Summary: This is an open-label multicenter, single-arm Phase II study of Fruquintinib in combination with FOLFIRI (leucovorin calcium (folinic acid), fluorouracil, and irinotecan) in participants with metastatic colorectal cancer (mCRC). The main goals of this study are to: * Evaluate the efficacy of the combination of fruquintinib + FOLFIRI in the 2nd-line mCRC setting * Evaluate the safety of the combina...
Related Latest Advances
Brand Information
- rescue after high-dose methotrexate therapy in adult and pediatric patients with osteosarcoma.
- diminishing the toxicity associated with overdosage of folic acid antagonists or impaired methotrexate elimination adult and pediatric patients.
- The treatment of adults with metastatic colorectal cancer in combination with fluorouracil.
- Increased gastrointestinal toxicities with fluorouracil
- Dermatologic: pruritus, rash
- Respiratory: dyspnea
- Other Clinical Events: temperature change, rigors, allergic reactions