Brand Name
Benznidazole
View Brand InformationFDA approval date: January 25, 2018
Form: Tablet
What is Benznidazole?
Benznidazole Tablets are indicated in pediatric patients 2 to 12 years of age for the treatment of Chagas disease caused by Trypanosoma cruzi. This indication is approved under accelerated approval based on the number of treated patients who became Immunoglobulin G antibody negative against the recombinant antigens of T. cruzi. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. Benznidazole Tablets, a nitroimidazole antimicrobial, is indicated in pediatric patients 2 to 12 years of age for the treatment of Chagas disease , caused by Trypanosoma cruzi . This indication is approved under accelerated approval based on the number of treated patients who became Immunoglobulin G antibody negative against the recombinant antigens of T. cruzi. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials .
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
A Randomized, Participant- and Investigator-blinded, Controlled, Parallel Group Study to Assess the Efficacy, Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of LXE408 in Participants With Chronic Chagas Disease Without Severe Organ Dysfunction.
Summary: This study is to investigate the ability of LXE408 to reduce or remove the level of parasites in the blood of people with chronic Chagas disease. Participants must have chronic Chagas disease without severe organ dysfunction.
Phase III Randomized,Multicenter Non-inferiority Study to Evaluate the Efficacy and Safety of Shorter Benznidazole Regimens Compared to the Standard Regimen to Treat Adult Patients With Chronic Chagas Disease
Summary: Chagas disease, a parasitic infection caused by Trypanosoma cruzi, is endemic in much of Latin America and affects people throughout the world. Currently treatment with the only two drugs effective against the infection, benznidazole and nifurtimox, has significant limitations including frequent adverse effects in adult patients. However, timely treatment is key to achieving global objectives of c...
Related Latest Advances
Brand Information
Benznidazole (benznidazole)
1INDICATIONS AND USAGE
Benznidazole Tablets are indicated in pediatric patients 2 to 12 years of age for the treatment of Chagas disease (American trypanosomiasis) caused by
This indication is approved under accelerated approval based on the number of treated patients who became Immunoglobulin G (IgG) antibody negative against the recombinant antigens of
2DOSAGE FORMS AND STRENGTHS
Benznidazole Tablets are available as 100 mg and 12.5 mg tablets.
The 100 mg white tablets are round and functionally scored twice as a cross on both sides, debossed with “E” on one side of each quarter portion.
The 12.5 mg white tablets are round and unscored, debossed with “E” on one side.
3ADVERSE REACTIONS
The following serious and otherwise important adverse reactions are discussed in greater detail in other sections of labeling:
- Potential for Genotoxicity, Carcinogenicity, and Mutagenicity
- Hypersensitivity Skin Reactions
- Central and Peripheral Nervous System Effects
- Hematological Manifestations of Bone Marrow Depression
3.1Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Benznidazole was evaluated in two randomized, double-blind, placebo-controlled trials (Trial 1
Trial 1 was conducted in pediatric patients 6 to 12 years of age with chronic indeterminate Chagas disease in Argentina. The chronic indeterminate form includes patients with serologic evidence of
Trial 2 was conducted in pediatric patients 7 to 12 years of age with chronic indeterminate Chagas disease in Brazil. A total of 129 patients were randomized to receive either benznidazole (7.5 mg/kg/day twice daily for 60 days; N = 64) or placebo (N = 65) and followed for 3 years.
Trial 3 was an uncontrolled study in pediatric patients 2 to 12 years of age with chronic indeterminate Chagas disease. A total of 37 pediatric patients with Chagas disease were enrolled in this safety and pharmacokinetics study. Patients were treated with benznidazole 5 to 8 mg/kg/day twice daily for 60 days.
Adverse Reactions Leading to Discontinuation
In Trial 1, benznidazole was discontinued due to an adverse reaction in 5/55 (9%) patients. Some patients had more than one adverse reaction resulting in treatment discontinuation. The adverse reactions included abdominal pain, nausea, vomiting, rash, decreased appetite, headache, and transaminases increased.
Common Adverse Reactions in Pediatric Patients
The most frequently reported adverse reactions in pediatric patients treated with benznidazole in Trial 1 were abdominal pain (25%), rash (16%), decreased weight (13%), and headache (7%).
In Trial 2, skin lesions were reported in 7 of 64 (11%) pediatric patients treated with benznidazole and in 2 of 65 patients receiving placebo. Adverse reactions reported in fewer than 5% of benznidazole-treated patients included nausea, anorexia, headache, abdominal pain and arthralgia.
In a subset of 19 pediatric patients 2 to 6 years of age treated with benznidazole in Trial 3, 6 patients (32%) had the following adverse reactions: rash, leukopenia, urticaria, eosinophilia, decreased appetite, and neutropenia. These adverse reactions were similar to those observed in the overall population of 37 patients.
3.2Postmarketing Experience
The following adverse reactions have been identified during the use of other formulations of benznidazole outside of the United States, or other nitroimidazole agents . Because these reactions are reported from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Other Formulations of Benznidazole:
Metronidazole, Another Nitroimidazole Agent, Structurally Related to Benznidazole
Cases of severe irreversible hepatotoxicity/acute liver failure, including cases with fatal outcomes with very rapid onset after initiation of systemic use of metronidazole, another nitroimidazole agent structurally related to benznidazole, have been reported in patients with Cockayne syndrome (latency from drug start to signs of liver failure as short as 2 days)
4DESCRIPTION
Benznidazole Tablets contain benznidazole, a nitroimidazole antimicrobial. The chemical name of benznidazole is N-benzyl-2-(2-nitro-1H-imidazol-1-yl) acetamide. The empirical formula is C
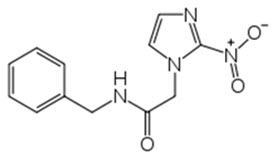
Figure 1: Benznidazole Structure
Benznidazole is a yellowish, practically crystalline powder that is practically insoluble in water, sparingly soluble in acetone and ethanol, and slightly soluble in methanol.
Benznidazole Tablets are white round tablets each containing 12.5 mg or 100 mg of benznidazole, for oral use. The 100 mg white tablets are round and functionally scored twice as a cross on both sides debossed with “E” on one side of each quarter portion. The 12.5 mg white tablets are round and unscored debossed with “E” on one side.
The inactive ingredients are as follows: magnesium stearate, NF; microcrystalline cellulose, NF; monohydrate lactose, NF; pre-gelatinized corn starch, NF; and sodium croscarmellose, NF.
5CLINICAL STUDIES
The safety and effectiveness of benznidazole for the treatment of Chagas disease in patients 6 to 12 years of age was established in two adequate and well-controlled trials (Trial 1 and Trial 2) as described below.
Trial 1 was a randomized, double-blind, placebo-controlled trial in children 6 to 12 years of age with chronic indeterminate Chagas disease conducted in Argentina. The chronic indeterminate form of Chagas disease includes patients with serologic evidence of
Trial 2 was a randomized, double-blind, placebo-controlled trial in pediatric patients 7 to 12 years of age with chronic indeterminate Chagas disease conducted in Brazil. A total of 129 patients were randomized to receive either benznidazole (7.5 mg/kg/day for 60 days) or placebo and followed for 3 years. Patients with three positive conventional serologic tests for antibodies to
Both trials measured antibodies by conventional and nonconventional assays. The nonconventional assays include F29-ELISA and AT- chemiluminescence-ELISA that are based on detection of anti-
In Trial 1 using conventional ELISA, 4 of 53 (7.5%) benznidazole subjects and 2 of 50 (4.0%) placebo subjects seroconverted to negative by the end of follow-up (difference 3.5, 95% CI (-7.0, 14.9)). In Trial 2 using conventional ELISA, 4 of 64 (6.3%) of benznidazole subjects and 0 of 65 placebo subjects seroconverted to negative by the end of follow-up (difference 6.3, 95% CI (0.3, 15.2)).
6REFERENCES
- Sosa Estani S, et al., 1998, Efficacy of Chemotherapy with Benznidazole in Children in the Indeterminate Phase of Chagas' Disease. Am J Trop Med Hyg 59: 526-529.
- de Andrade, Ana Lucia S. Sgambatti, et al., 1996, Randomised Trial of Efficacy of Benznidazole in Treatment of Early
- Altcheh, Jaime, et al., 2014, Population Pharmacokinetic Study of Benznidazole in Pediatric Chagas Disease Suggests Efficacy Despite Lower Plasma Concentrations than in Adults. PLoS Negl Trop Dis 8:e2907.
- García-Bournissen, F, S Moroni, ME Marson, et al., 2015, Limited Infant Exposure to Benznidazole Through Breast Milk During Maternal Treatment for Chagas Disease. Arch Dis Child 100:90-94.
- Bernacchi, AS, CR de Castro, EG de Toranzo, and JA Castro, 1986, Effects of Nifurtimox or Benznidazole Administration on Rat Testes: Ultrastructural Observations and Biochemical Studies, Exp Mol Pathol 45: 245-256.
- Vieira, CL, TL Lamano-Carvalho, AL Favaretto, MM Valenca, J Antunes-Rodridgues, and AA Barreira, 1989, Testes Alterations in Pubertal Benznidazole-treated Rats, Braz J Med Biol Res, 22: 695-698.
- Navarro, ML and R Nagel, 1990, Abnormal Sperm Induced in Mice by Oral Administration of Antichagasic Drugs, Comunicaciones Biologicas, 8: 251-258.
- de Castro, RC, EG Diaz de Toranzo, and JA Castro, 1992, Benznidazole-induced Ultrastructural Alterations in Rat Adrenal Cortex: Mechanistic Studies, Toxicology, 74: 223-232.
- Diaz, EG, RC de Castro, M Montalto de Mecca, and JA Castro, 2000, Benznidazole-induced Ultrastructural and Biochemical Alterations in Rat Colon, Acta Pharmacol Sin, 21: 961-966.
- de Castro, RC, M Montalto de Mecca, SL Fanelli, EC de Ferreyra, EG Diaz, and JA Castro, 2003, Benznidazole-induced Ultrastructural and Biochemical Alterations in Rat Esophagus, Toxicology, 191: 189-198.
- de Castro, CR, EGD de Toranzo, AS Bernacchi, M Carbone, and JA Castro, 1989, Ultrastructural Alterations in Ovaries from Nifurtimox or Benznidazole-treated Rats: Their Relation to Ovarian Nitroreductive Biotransformation of Both Drugs, Exp Mol Pathol, 50: 385-397.
- Scharer, K, 1972, Selective Purkinje Cell Damage in Dogs after Oral Administration of High Doses of Nitroimidazole Derivatives, Verhandlungen der Deutschen Gesellschaft fur Pathologie, 56: 407-10.
- Flores-Vieira, CLL and AA Barreira, 1997a, Experimental Benznidazole Encephalopathy: I. Clinical-Neurological Alterations, J Neurol Sci, 150: 3-11.
- Flores-Vieira, CLL, L Chimelli, RMF Fernandez, and AA Barreira, 1997b, Experimental Benznidazole Encephalopathy: II. Electroencephalographic and Morphological Alterations, J Neurol Sci, 150:13-25.
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential that exposure to Benznidazole Tablets during pregnancy can result in fetal harm.
Advise females to inform their healthcare provider of a known or suspected pregnancy
Advise females of reproductive potential to use effective contraception while taking
Benznidazole Tablets and for 5 days after the last dose
Lactation
Advise women not to breastfeed during treatment with Benznidazole Tablets
Infertility
Advise males of reproductive potential that Benznidazole Tablets may impair fertility
Important Administration Instructions
Advise patients and parents/caregivers of pediatric patients taking Benznidazole Tablets that:
•Benznidazole Tablets 100 mg are functionally scored tablets which can be split into one-half (50 mg) or one-quarter (25 mg) at the scored lines to provide doses less than 100 mg.
•Benznidazole Tablets 12.5 mg and 100 mg (whole or split) can be made into a slurry in a specified volume of water for the pediatric population
Hypersensitivity Skin Reactions
Advise patients that serious skin reactions can occur with Benznidazole Tablets. In case of skin reactions, presenting with additional symptoms of systemic involvement such as lymphadenopathy, fever and/or purpura, discontinuation of treatment is necessary.
Central and Peripheral Nervous System Effects
Advise patients that treatment can potentially cause paresthesia or symptoms of peripheral neuropathy. In cases where neurological symptoms occur, immediate discontinuation of treatment is recommended.
Hematological Manifestations of Bone Marrow Depression
Advise patients that there have been hematological manifestations of bone marrow depression, such as anemia and leukopenia, which are reversible, and normalized after treatment discontinuation.
Manufactured for Chemo Research, S.L.
Manufactured by Laboratorios Liconsa S.A.
Distributed by:

Exeltis USA, Inc.
8INSTRUCTIONS FOR USE
BENZNIDAZOLE
tablets, for oral use
tablets, for oral use
Read this Instructions for Use before you start taking BENZNIDAZOLE and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
Note:
• Your doctor may need to change your dose of BENZNIDAZOLE during treatment as needed.
• BENZNIDAZOLE 100 mg tablets can be taken whole or broken at scored lines.
• BENZNIDAZOLE 100 mg tablets are marked with scored lines and may be broken at these scored lines to provide the following doses: 75 mg, 50 mg and 25 mg.
100 mg treatment (take the whole tablet)
How to break your BENZNIDAZOLE 100 mg tablet:
• Hold the tablet between your thumbs and index fingers close to the scored line (
• Apply enough pressure to break the tablet at the scored line (
• Only use a tablet that has been broken at the scored line (
•
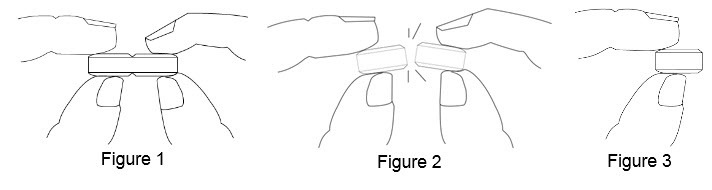
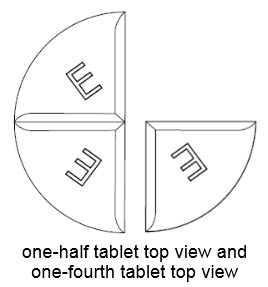
75 mg treatment (take one-half of the tablet and one-fourth of the tablet)
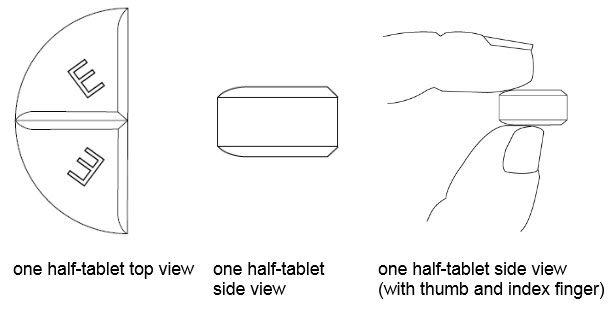
50 mg treatment (take one-half of the tablet)
25 mg treatment (take one-fourth of the tablet)
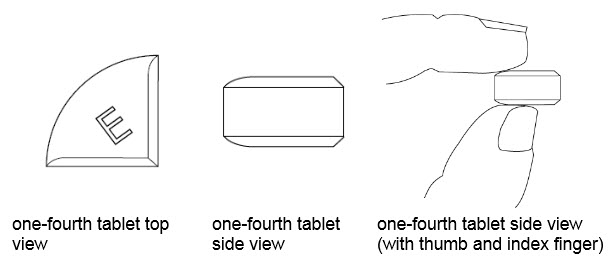
How should I store BENZNIDAZOLE?
• Store BENZNIDAZOLE at room temperature 20° to 25°C (68°to 77°F).
• Keep BENZNIDAZOLE in the bottle that it comes in and keep the bottle tightly closed.
• Keep BENZNIDAZOLE away from moisture.
Keep BENZNIDAZOLE and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by: Laboratori
Issued: August 2017
9PRINCIPAL DISPLAY PANEL - 100 mg CARTON

NDC 0642-7464-10
Benznidazole tablets
For oral use
Exeltis
Rethinking healthcare
Rethinking healthcare
Keep out of the reach of children.
Store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Manufactured for Chemo Research, S.L.,
Manufactured by Laboratorios Liconsa S.A.,
Distributed by Exeltis USA, Inc.,
1-877-324-9349 www.exeltisusa.com
Rev: 09/2025
See prescribing information
Benznidazole
Benznidazole tablets
Lot No.:
10PRINCIPAL DISPLAY PANEL - 12.5 mg CARTON

NDC 0642-7463-12
Benznidazole tablets
For oral use
Exeltis
Rethinking healthcare
Rethinking healthcare
Keep out of the reach of children.
Store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Manufactured for Chemo Research, S.L.,
Manufactured by Laboratorios Liconsa S.A.,
Distributed by Exeltis USA, Inc.,
1-877-324-9349 www.exeltisusa.com
Rev: 09/2025
See prescribing information
Benznidazole
Benznidazole tablets
Lot No.:

