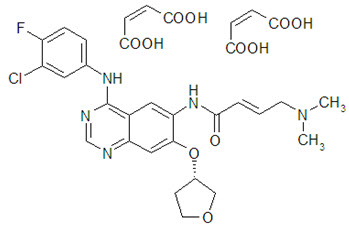
Gilotrif
What is Gilotrif (Afatinib)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Despite recent advances, the prognosis of patients with advanced gastric cancer remains poor. At present, regimens that combine a platinum and fluorouracil agent either alone or in combination with a third drug such as epirubicin or taxane constitute the most effective treatment option in the first-line metastatic setting, resulting in a median OS of approximately 10 months. In the second-line set...
Summary: The goal of this clinical trial is to learn if Afatinib plus Palbociclib works in previously treated recurrent or metastatic esophageal squamous cell carcinoma. It will also learn about the safety of the combination of Afatinib and Palbociclib. The main questions it aims to answer are: What is the safe and tolerable dose of Afatinib when combined with 100 mg of Palbociclib (administered orally, th...
Summary: Global, Phase 3, randomized, multicenter, open-label study evaluating the efficacy and safety of firmonertinib at a dose level of 240 mg QD compared to investigator's choice of osimertinib (80 mg QD) or afatinib (40 mg QD) in participants who have locally advanced or metastatic NSCLC with EGFR PACC mutations, and who have not received any prior therapy for advanced disease. Participants will be ra...
Related Latest Advances
Brand Information
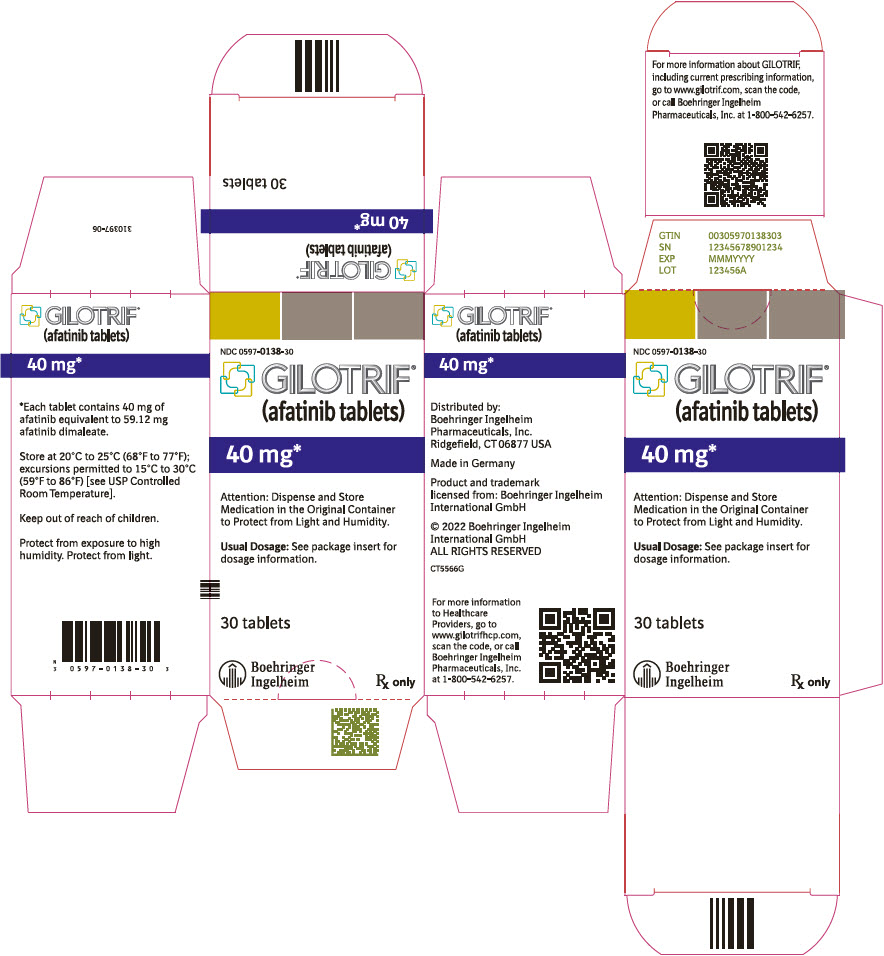
- 40 mg tablets: light blue, film-coated, round, biconvex, bevel-edged tablets debossed with "T40" on one side and the Boehringer Ingelheim company symbol on the other side.
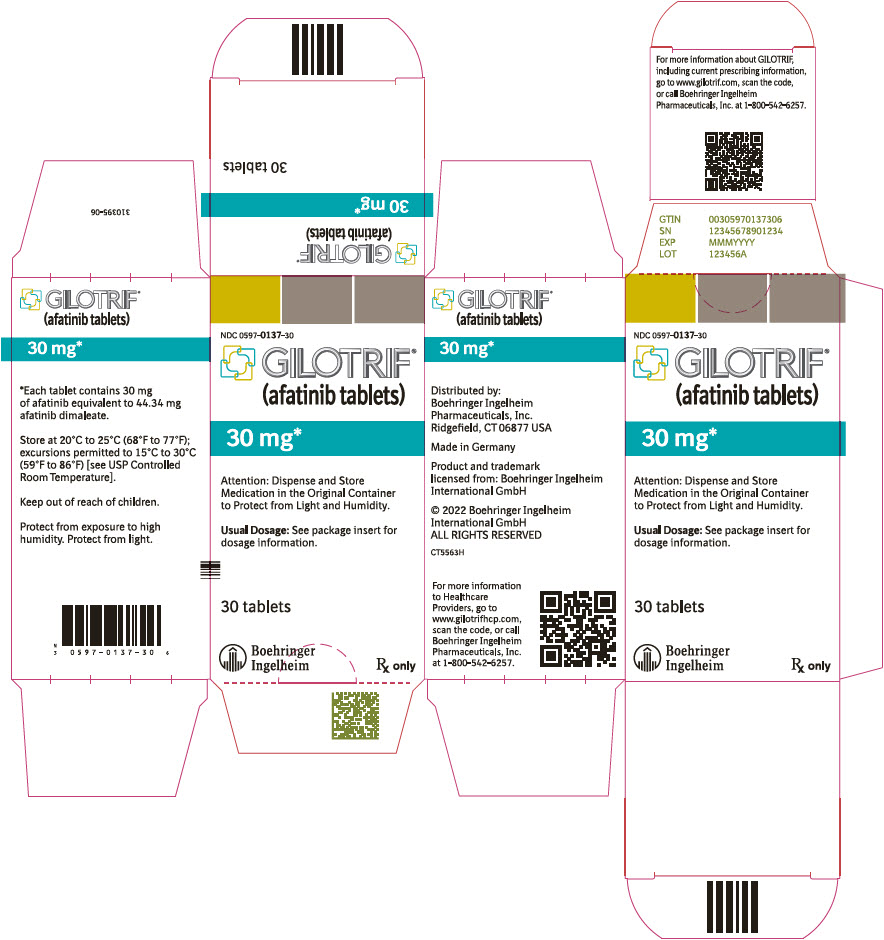
- 30 mg tablets: dark blue, film-coated, round, biconvex, bevel-edged tablets debossed with "T30" on one side and the Boehringer Ingelheim company symbol on the other side.
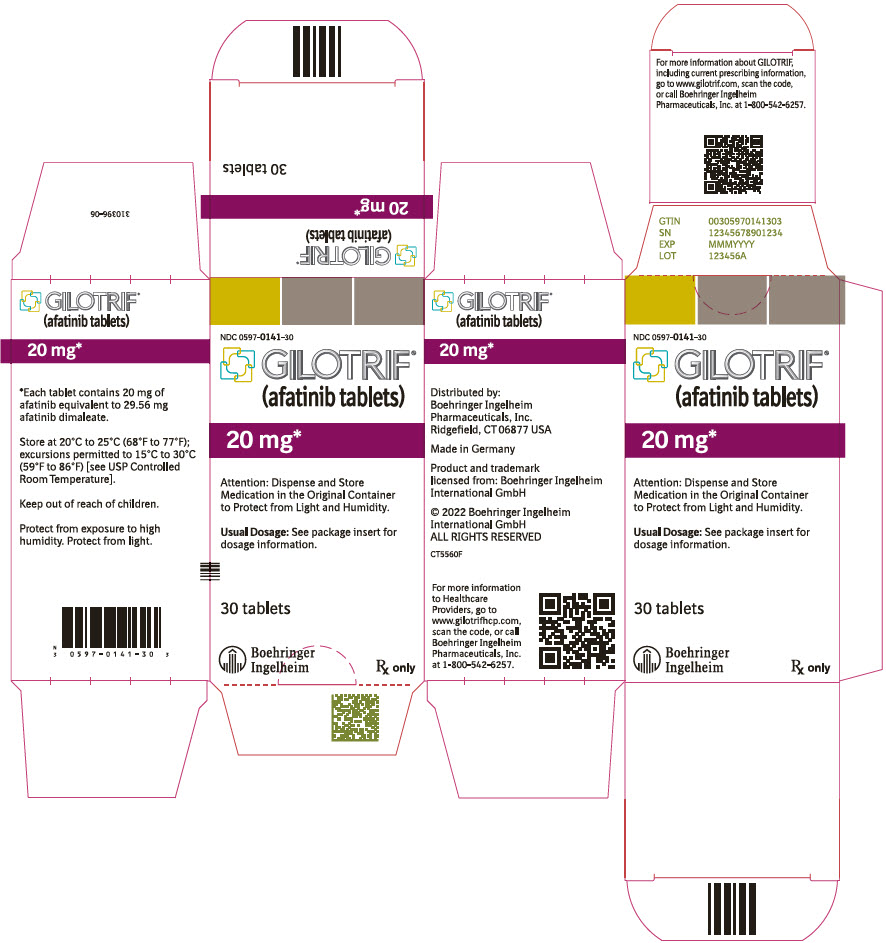
- 20 mg tablets: white to slightly yellowish, film-coated, round, biconvex, bevel-edged tablets debossed with "T20" on one side and the Boehringer Ingelheim company symbol on the other side.
- Diarrhea
- Bullous and Exfoliative Skin Disorders
- Interstitial Lung Disease
- Hepatic Toxicity
- Gastrointestinal Perforation
- Keratitis
- Pancreatitis
- Toxic epidermal necrolysis/Stevens Johnson syndrome