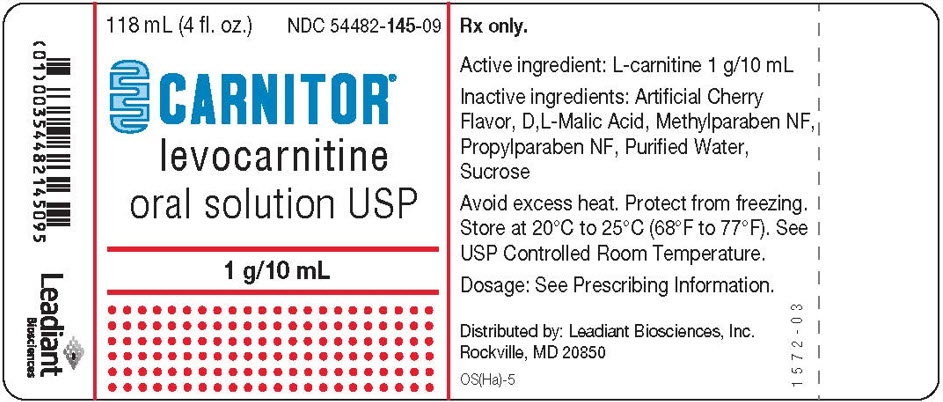
Carnitor
What is Carnitor (Levocarnitine)?
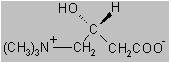
Fatigue, muscle weakness or difficulty recovering from illness can sometimes stem from a problem deep within our body’s energy system, how our cells use fat as fuel. Levocarnitine (also known as L-carnitine) is a naturally occurring nutrient and medication that helps transport fatty acids into cells, where they can be converted into usable energy.
Doctors prescribe Levocarnitine to individuals who cannot produce or maintain enough of it naturally such as those with carnitine deficiency, chronic kidney disease or certain metabolic disorders. It plays a key role in improving energy levels, muscle function and overall well-being. Levocarnitine has been used for decades and is available in both oral and injectable forms.
What does Levocarnitine do?
Levocarnitine helps the body convert fat into energy, a process vital for the heart, muscles and brain. It is primarily used to treat primary and secondary carnitine deficiency, conditions where the body cannot make or maintain adequate carnitine levels.
Doctors may prescribe it for:
- Genetic carnitine deficiency disorders, where enzyme or transporter defects prevent carnitine production.
- Patients with chronic kidney disease on dialysis, who lose carnitine through the treatment process.
- Individuals with certain liver or metabolic conditions, where energy metabolism is impaired.
By restoring normal carnitine levels, Levocarnitine helps reduce fatigue, muscle cramps and weakness, improving physical endurance and overall energy. In some studies, patients with kidney-related deficiencies reported better muscle strength and fewer symptoms of tiredness after consistent therapy (NIH, 2023).
How does Levocarnitine work?
Levocarnitine acts like a transport shuttle that carries long-chain fatty acids into the mitochondria, the cell’s “power plants” where these fats are broken down to generate energy. Without enough carnitine, fats build up in tissues instead of being used efficiently, which can cause weakness, poor metabolism and organ dysfunction.
Clinically, this mechanism is crucial because it helps the heart and skeletal muscles, organs that rely heavily on fat for energy, function optimally. By improving the use of fat as fuel, Levocarnitine can enhance stamina and reduce symptoms of metabolic stress.
Doctors sometimes describe it as “recharging the body’s energy pathways,” particularly in patients whose conditions or medications disrupt normal metabolism.
Levocarnitine side effects
Levocarnitine is generally well tolerated, but some patients may experience mild side effects. Most are temporary and improve as the body adjusts to the medication.
Common side effects include:
- Nausea or vomiting
- Diarrhea or stomach cramps
- Unusual body odor (a “fishy” smell sometimes occurs due to carnitine metabolism)
Less common but possible effects:
- Headache or dizziness
- Muscle weakness (especially in patients with uremia)
- Low blood sugar, particularly in individuals with diabetes
Serious reactions are rare, but patients should seek medical help if they experience chest pain, irregular heartbeat, severe weakness or allergic reactions such as rash or swelling.
Levocarnitine may not be suitable for people with known hypersensitivity to the compound or those with certain metabolic conditions that cause high blood ammonia levels. Always discuss your full medical history with your healthcare provider before starting therapy (FDA, 2024).
Levocarnitine dosage
Levocarnitine comes in several forms: oral tablets, liquid solution and injections. The exact dose and route depend on the cause and severity of the deficiency, age and body weight.
For most patients, therapy begins under medical supervision, and the dosage may be gradually adjusted based on response and lab results. Physicians often monitor:
- Blood carnitine levels to ensure optimal dosing
- Kidney and liver function to assess tolerance
- Muscle strength and fatigue patterns as indicators of clinical improvement
Regular follow-up visits help doctors fine-tune treatment and prevent side effects. Patients on dialysis typically receive injections during or after sessions under medical guidance.
Special care is taken in older adults or those with heart or liver disease to avoid overcorrection or accumulation of the compound.
Does Levocarnitine have a generic version?
Yes. Levocarnitine is available in both generic and brand-name forms. Common brand names include Carnitor® and Carnitor SF®. The generic form, levocarnitine, is FDA-approved and considered equally safe and effective as the branded version.
Generic options are often more affordable and widely available in both oral and injectable preparations, making ongoing treatment easier for patients requiring long-term supplementation.
Conclusion
Levocarnitine is a trusted and effective therapy that supports the body’s energy metabolism, particularly for individuals whose conditions prevent them from producing enough carnitine naturally. By restoring balance to how the body converts fat into fuel, it helps improve strength, stamina, and overall quality of life.
While most people tolerate it well, close medical supervision ensures the right dose and ongoing safety. Patients should always follow their doctor’s instructions and report any unusual symptoms promptly.
When prescribed and monitored by a qualified healthcare professional, Levocarnitine offers a safe and reliable way to help the body maintain its vital energy processes and enhance daily well-being.
References
- U.S. Food and Drug Administration (FDA). (2024). Levocarnitine prescribing information. Retrieved from https://www.fda.gov
- National Institutes of Health (NIH). (2023). Carnitine: Fact sheet for health professionals. Retrieved from https://ods.od.nih.gov
- Mayo Clinic. (2023). Levocarnitine (oral route, intravenous route) description and brand names. Retrieved from https://www.mayoclinic.org
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- Bohmer, T., Rydning, A. and Solberg, H.E. 1974. Carnitine levels in human serum in health and disease.
- Brooks, H., Goldberg, L., Holland, R.
- Christiansen, R., Bremer, J. 1976. Active transport of butyrobetaine and carnitine into isolated liver cells.
- Lindstedt, S. and Lindstedt, G. 1961. Distribution and excretion of carnitine in the rat.
- Rebouche, C.J. and Engel, A.G. 1983. Carnitine metabolism and deficiency syndromes.
- Rebouche, C.J. and Paulson, D.J. 1986. Carnitine metabolism and function in humans.
- Scriver, C.R., Beaudet, A.L., Sly, W.S. and Valle, D. 1989.
- Schaub, J., Van Hoof, F. and Vis, H.L. 1991.
- Marzo, A., Arrigoni Martelli, E., Mancinelli, A., Cardace, G., Corbelletta, C., Bassani, E. and Solbiati, M. 1991. Protein binding of L-carnitine family components.
- Rebouche, C.J. 1991. Quantitative estimation of absorption and degradation of a carnitine supplement by human adults.

Date of Issue: 07/23 OPI-16
(levocarnitine) Oral Solution