Generic Name
Aspart
Brand Names
Remodulin, NovoLog, Yutrepia, Treprostinil, Orenitram, Tyvaso DPI, Tyvaso, Merilog, FiAsp
FDA approval date: August 27, 2001
Classification: Prostacycline Vasodilator
Form: Injection, Inhalant, Tablet, Kit, Capsule
What is Remodulin (Aspart)?
Treprostinil injection is a prostacyclin mimetic indicated for: Treatment of pulmonary arterial hypertension to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH , PAH associated with congenital systemic-to-pulmonary shunts , or PAH associated with connective tissue diseases .
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Remodulin (treprostinil)
1DOSAGE FORMS AND STRENGTHS
20-mL vial containing 2 mg treprostinil (0.1 mg per mL).
20-mL vial containing 4 mg treprostinil (0.2 mg per mL).
20-mL vial containing 8 mg treprostinil (0.4 mg per mL).
20-mL vial containing 20 mg treprostinil (1 mg per mL).
20-mL vial containing 50 mg treprostinil (2.5 mg per mL).
20-mL vial containing 100 mg treprostinil (5 mg per mL).
20-mL vial containing 200 mg treprostinil (10 mg per mL).
20-mL vial containing 400 mg treprostinil (20 mg per mL).
2CONTRAINDICATIONS
None
3ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in labeling: Infections associated with intravenous administration
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
3.2Post-Marketing Experience
In addition to adverse reactions reported from clinical trials, the following events have been identified during post-approval use of Remodulin. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The following events have been chosen for inclusion because of a combination of their seriousness, frequency of reporting, and potential connection to Remodulin. These events are thrombophlebitis associated with peripheral intravenous infusion, thrombocytopenia, bone pain, pruritus, dizziness, arthralgia, myalgia/muscle spasm, and pain in extremity. In addition, generalized rashes, sometimes macular or papular in nature, and cellulitis have been infrequently reported.
4OVERDOSAGE
Signs and symptoms of overdose with Remodulin during clinical trials are extensions of its dose-limiting pharmacologic effects and include flushing, headache, hypotension, nausea, vomiting, and diarrhea. Most events were self-limiting and resolved with reduction or withholding of Remodulin.
In controlled clinical trials using an external infusion pump, seven patients received some level of overdose and in open-label follow-on treatment seven additional patients received an overdose; these occurrences resulted from accidental bolus administration of Remodulin, errors in pump programmed rate of administration, and prescription of an incorrect dose. In only two cases did excess delivery of Remodulin produce an event of substantial hemodynamic concern (hypotension, near-syncope).
One pediatric patient was accidentally administered 7.5 mg of Remodulin via a central venous catheter. Symptoms included flushing, headache, nausea, vomiting, hypotension, and seizure-like activity with loss of consciousness lasting several minutes. The patient subsequently recovered.
5DESCRIPTION
Remodulin (treprostinil) Injection is a sterile solution of treprostinil, a prostacyclin mimetic, formulated for subcutaneous or intravenous administration. Remodulin is supplied in 20-mL multidose vials in eight strengths, containing 2 mg (0.1 mg/mL), 4 mg (0.2 mg/mL), 8 mg (0.4 mg/mL), 20 mg (1 mg/mL), 50 mg (2.5 mg/mL), 100 mg (5 mg/mL), 200 mg (10 mg/mL), or 400 mg (20 mg/mL) of treprostinil. Each mL also contains 5.3 mg sodium chloride (except for the 10 mg/mL and 20 mg/mL strengths, which contain 4.0 mg sodium chloride), 3 mg metacresol, 6.3 mg sodium citrate dihydrate, and water for injection. Sodium hydroxide and hydrochloric acid may be added to adjust pH between 6.0 and 7.2.
Treprostinil is chemically stable at room temperature and neutral pH.
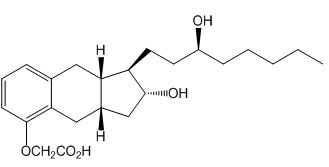
Treprostinil is (1
The structural formula of treprostinil is:
Sterile Diluent for Remodulin is a high-pH (pH~10.4) glycine diluent supplied in a 50-mL vial containing 50 mL of Sterile Diluent for Remodulin. Each vial contains 94 mg glycine, 73.3 mg sodium chloride, sodium hydroxide (to adjust pH), and water for injection.
6HOW SUPPLIED/STORAGE AND HANDLING
Remodulin is supplied in 20-mL multidose vials as sterile solutions in water for injection, individually packaged in cartons. Unopened vials of Remodulin are stable until the date indicated when stored at 25°C (77°F), with excursions permitted to 2-30°C (36-86°F). A single vial of Remodulin should be used for no more than 30 days after the initial introduction into the vial.
Remodulin Injection is supplied as:
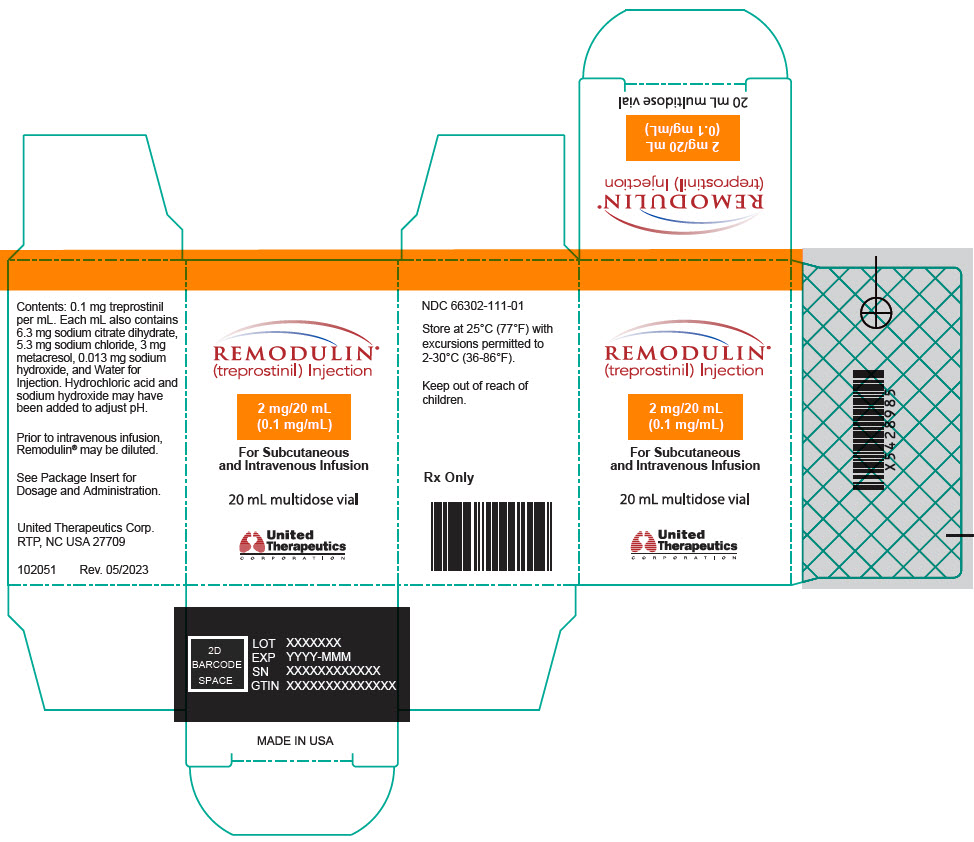
7PRINCIPAL DISPLAY PANEL - 0.1 mg/mL Vial Carton
REMODULIN
2 mg/20 mL
For Subcutaneous
20 mL multidose vial
United
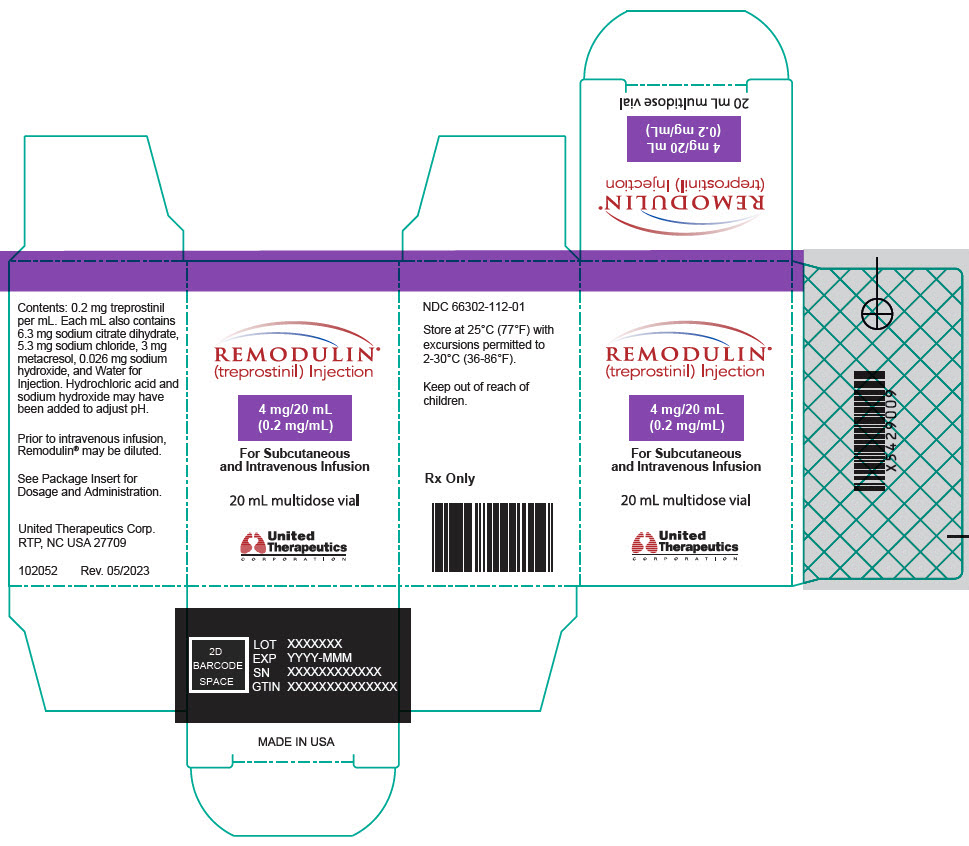
8PRINCIPAL DISPLAY PANEL - 0.2 mg/mL Vial Carton
REMODULIN
4 mg/20 mL
For Subcutaneous
20 mL multidose vial
United
9PRINCIPAL DISPLAY PANEL - 0.4 mg/mL Vial Carton
REMODULIN
8 mg/20 mL
For Subcutaneous
20 mL multidose vial
United
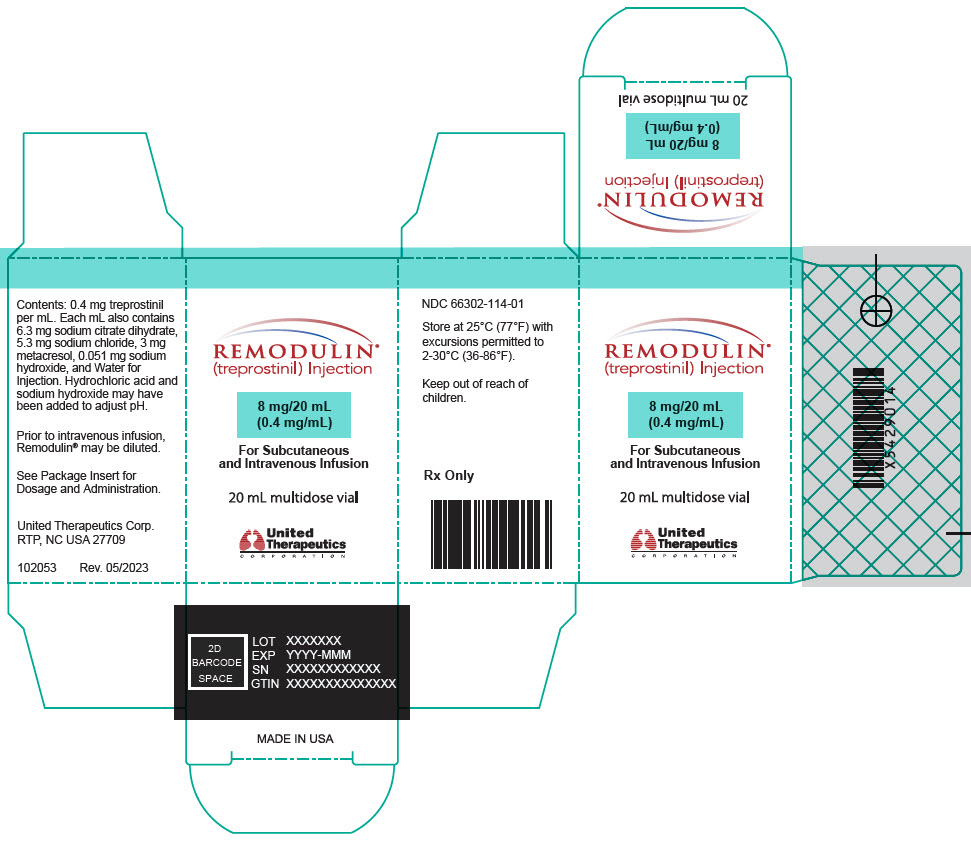
10PRINCIPAL DISPLAY PANEL - 1 mg/mL Vial Carton
REMODULIN
20 mg/20 mL
For Subcutaneous
20 mL multidose vial
United
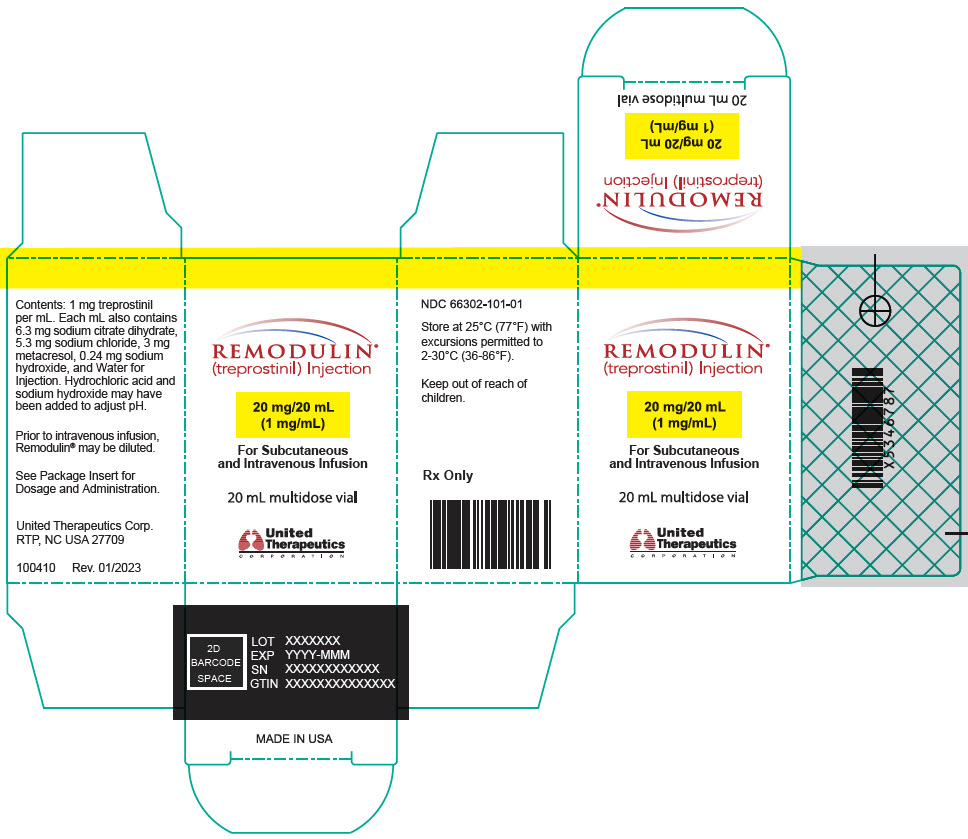
11PRINCIPAL DISPLAY PANEL - 2.5 mg/mL Vial Carton
REMODULIN
50 mg/20 mL
For Subcutaneous
20 mL multidose vial
United
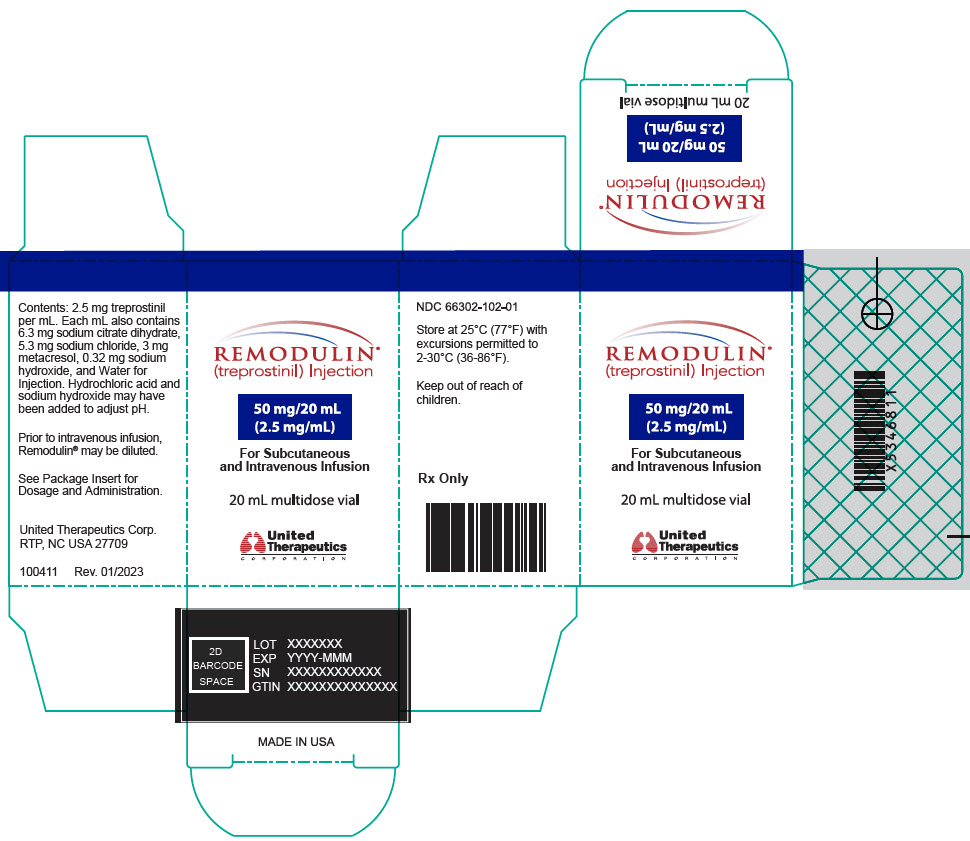
12PRINCIPAL DISPLAY PANEL - 5 mg/mL Vial Carton
REMODULIN
100 mg/20 mL
For Subcutaneous
20 mL multidose vial
United
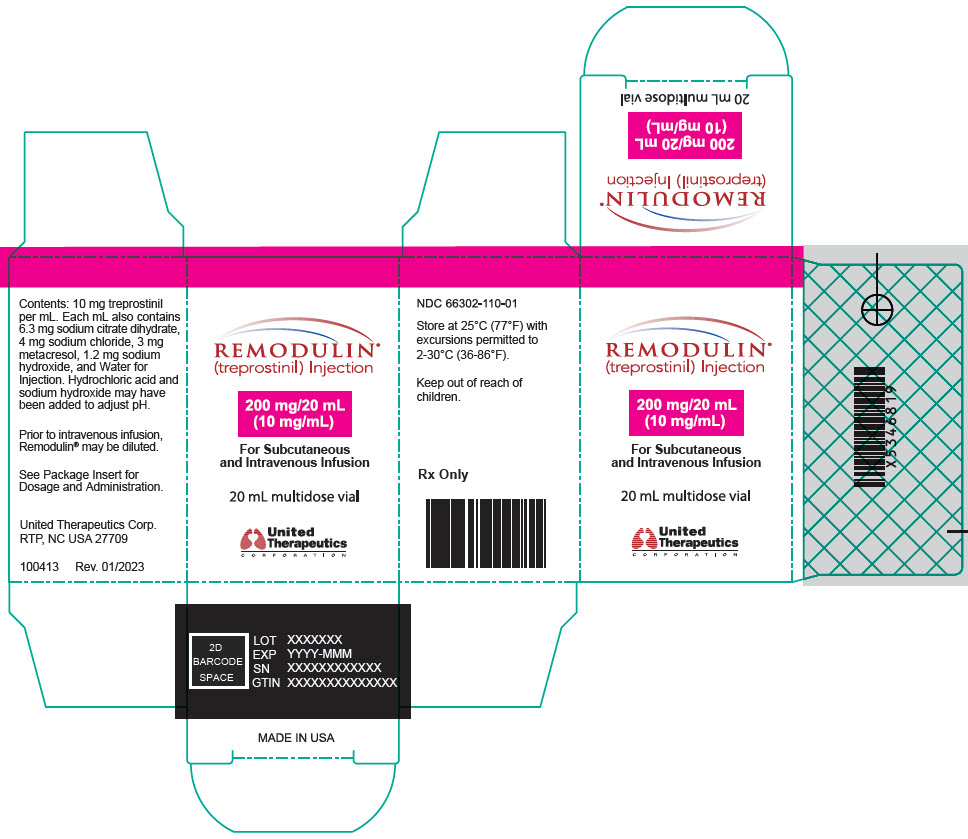
13PRINCIPAL DISPLAY PANEL - 10 mg/mL Vial Carton
REMODULIN
200 mg/20 mL
For Subcutaneous
20 mL multidose vial
United
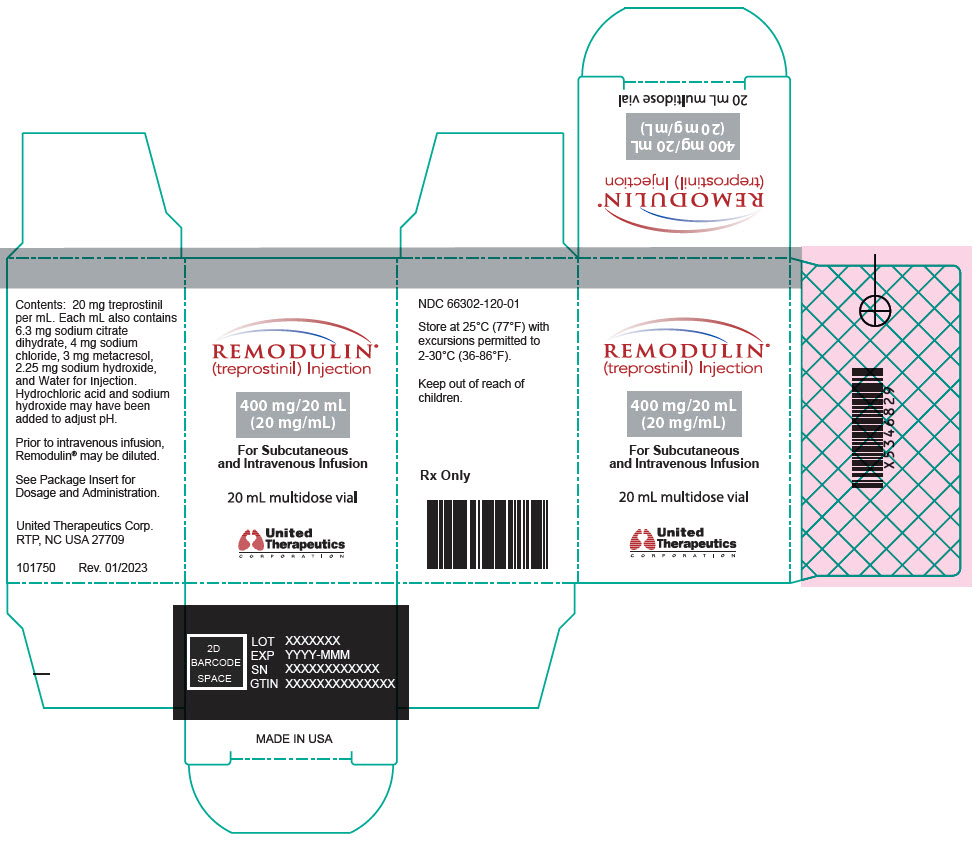
14PRINCIPAL DISPLAY PANEL - 20 mg/mL Vial Carton
REMODULIN
400 mg/20 mL
For Subcutaneous
20 mL multidose vial
United
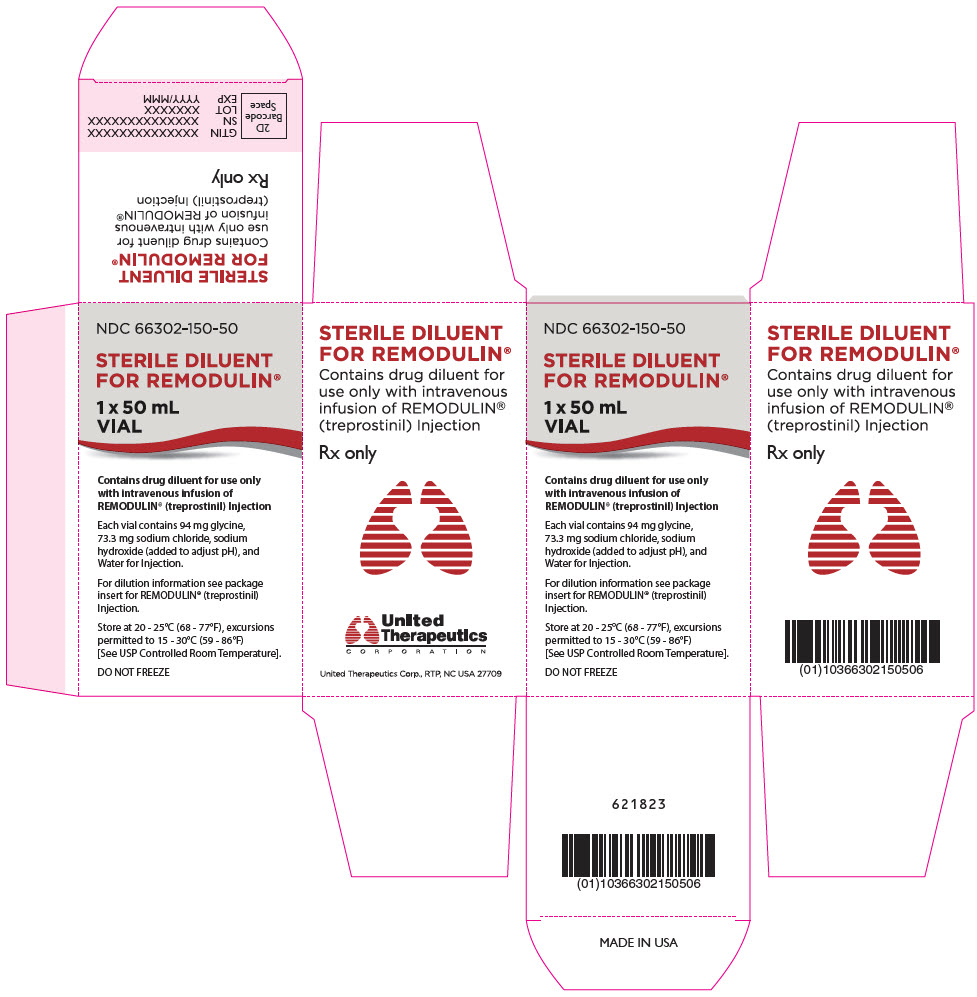
15PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton
NDC 66302-150-50
STERILE DILUENT
1 x 50 mL
Contains drug diluent for use only
Each vial contains 94 mg glycine,
For dilution information see package
Store at 20 - 25°C (68 - 77°F), excursions
DO NOT FREEZE