Impavido
What is Impavido (Miltefosine)?
Approved To Treat
Related Clinical Trials
Summary: randomised control clinical trial to evaluate miltefosine, thermotherapy and the combination miltefosine-thermotherapy are effective, safe and tolerable alternative treatment options to treat cutaneous leishmaniasis caused by L. tropica, in Pakistan compared to the standard of care.
Summary: This is a phase 2 study of the combination of inhaled-pentamidine plus oral miltefosine for Bolivian mucosal leishmaniasis.
Summary: While there are indications that 28 days of miltefosine is not sufficient for treating CL by L. aethiopica, a better understanding of what happens in terms of parasite clearance and drug dosing is lacking. In this study, longitudinal measurements of parasite and drug concentrations during treatment are done to monitor parasite kinetics as well as pharmacokinetics. This data will be crucial to prov...
Related Latest Advances
Brand Information
- Pregnancy: IMPAVIDO is contraindicated in pregnancy. Based on animal data, miltefosine may cause fetal harm [see Contraindications (.
- Females of Reproductive Potential: Verify pregnancy status prior to initiating IMPAVIDO. To prevent pregnancy, females of reproductive potential should use effective contraception during treatment and for 5 months after the last dose [see Dosage and Administration (
- Visceral leishmaniasis caused by
- Cutaneous leishmaniasis caused by
- Mucosal leishmaniasis caused by
- Leishmania species studied in clinical trials evaluating IMPAVIDO were based on epidemiologic data [see Clinical Trials (.
- There may be geographic variation in clinical response of the same
- The efficacy of IMPAVIDO in the treatment of other
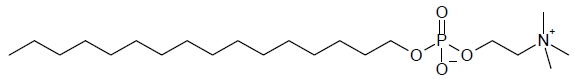
- IMPAVIDO is administered with food to ameliorate gastrointestinal side effects.
- Instruct the patient to swallow the capsule whole and not to chew it or break it apart. Instruct the patient to complete the full course of therapy.
- Inform the patient that abdominal pain, nausea, vomiting, and diarrhea are common side effects of therapy with IMPAVIDO and instruct the patient to inform their healthcare provider if these gastrointestinal side effects are severe or persistent. Instruct the patient to consume sufficient fluids to avoid dehydration and, consequently, the risk of kidney injury.
- Advise pregnant women and females of reproductive potential that IMPAVIDO may cause fetal harm. Advise females to inform their healthcare provider of a known of suspected pregnancy
- There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to IMPAVIDO during pregnancy
- Advise women not to breastfeed during treatment with IMPAVIDO and for 5 months after the last dose
- Advise women of reproductive potential to use effective contraception during treatment with IMPAVIDO and for 5 months after the last dose
- Advise women who use oral contraceptives to use an additional non-oral method of effective contraception during IMPAVIDO therapy, if vomiting and/or diarrhea occurs
- Advise women who become pregnant while being treated with IMPAVIDO, to discontinue treatment with IMPAVIDO and seek counseling from their healthcare provider about the potential risk to the fetus
- Advise male patients that semen quality and sperm parameters may be adversely affected by treatment with IMPAVIDO. Advise male patients that the effects of IMPAVIDO on spermatogenesis may persist for an unknown duration of time
- Advise females and males of reproductive potential that IMPAVIDO may impair fertility.
- Advise male patients that diminution in ejaculate volume, including temporary absence of ejaculate, and scrotal tenderness may occur during treatment with IMPAVIDO. Male patients should report any concerning genitourinary symptoms to their healthcare provider


(miltefosine) capsules
50 mg per Capsule
magnesium stearate, microcrystalline cellulose, talc. The capsule shell
contains gelatin, titanium dioxide, ferric oxide and purified water.
prescribing information.
50 mg per Capsule



