Pulmozyme
What is Pulmozyme (Dornase)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Germ cell tumors (GCTs) are highly curable malignancies; however, a subset of patients with relapsed or refractory disease after first- and second-line chemotherapy have a very poor prognosis, with long-term survival rates below 5%. New therapeutic strategies are needed in this setting. Emerging evidence indicates that extracellular DNA and markers of NETosis are associated with poor prognosis in ...
Summary: Severe hypoxemia following trauma may happen in many circumstances (aspiration, ventilation-associated pneumonia, lung contusion...), most of which are not exclusively associated with a direct injury to the lungs. Severe trauma and associated musculoskeletal injuries result in the acute release of Damage-Associated Molecular Patterns (DAMPs) in plasma, many of which are made of nucleic acids. DAMP...
Summary: Patients presenting to the emergency department with acute ischemic stroke, who are are eligible for standard intravenous thrombolytic therapy within 4.5 hours of stroke onset will be assessed for major vessel occlusion to determine their eligibility for the trial. All participants will receive intravenous tenecteplase (or alteplase due to manufacturer shortage) and endovascular thrombectomy as st...
Related Latest Advances
Brand Information
- 30 unit cartons containing 5 foil pouches of 6 single-dose ampules. Each 2.5 mL ampule contains 2.5 mg of dornase alfa (1 mg/mL): NDC 50242-100-40.
- to clean the nebulizer before first use and after each use as recommended.
- to disinfect the nebulizer parts by using the disinfecting method recommended.
- to replace nebulizer parts as recommended.
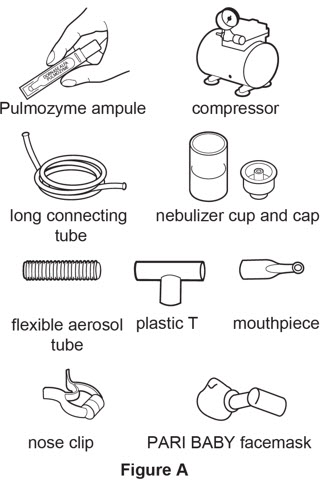
- 1 Pulmozyme ampule
- Compressor
- Nebulizer cup and cap (screw-on or snap-on)
- Plastic T (not needed for Sidestream nebulizer or PARI BABY)
- Flexible aerosol tube (not needed for Sidestream nebulizer or PARI BABY)
- Mouthpiece (clean) or PARI BABY facemask
- Long connecting tube
- Nose clip (optional, not needed for PARI BABY)
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
US License No. 1048
- to clean the nebulizer before first use and after each use as recommended
- to disinfect the nebulizer parts by using the disinfecting method recommended
- to replace nebulizer parts as recommended
- 1 Pulmozyme ampule (See
- Vibrating mesh nebulizer and its parts
- Manufacturer's instruction manual for the vibrating mesh nebulizer
- Nose clip (optional) (See
- Store Pulmozyme ampules at a refrigerated temperature between 36°F to 46°F (2°C to 8°C) in their protective foil pouch to protect from light and heat until you are ready to use them. When the protective foil pouch is opened, the unused ampules must be kept refrigerated in the protective foil pouch to protect from light and heat.
- When traveling, Pulmozyme ampules should be kept refrigerated in their protective foil pouch to protect from light and heat.
- Protect Pulmozyme from excessive heat and light.
- Do not use Pulmozyme if the ampules have been exposed to room temperature at 72°F to 82°F (22°C to 28°C) for more than a total of 60 hours or if the solution has turned cloudy or discolored.
- Do not use Pulmozyme past the expiration date printed on the ampule.