Brand Name
Aptivus
Generic Name
Tipranavir
View Brand Information FDA approval date: July 01, 2005
Classification: Protease Inhibitor
Form: Capsule
What is Aptivus (Tipranavir)?
APTIVUS, co-administered with ritonavir, is indicated for combination antiretroviral treatment of HIV-1 infected patients who are treatment-experienced and infected with HIV-1 strains resistant to more than one protease inhibitor . This indication is based on analyses of plasma HIV-1 RNA levels in two controlled studies of APTIVUS/ritonavir of 48 weeks duration in treatment-experienced adults and one open-label 48-week study in pediatric patients age 2 to 18 years. The adult studies were conducted in clinically advanced, 3-class antiretroviral treatment-experienced adults with evidence of HIV-1 replication despite ongoing antiretroviral therapy. The following points should be considered when initiating therapy with APTIVUS/ritonavir: The use of APTIVUS/ritonavir in treatment-naïve patients is not recommended [ see Warnings and Precautions.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Aptivus (tipranavir)
1INDICATIONS AND USAGE
APTIVUS, co-administered with ritonavir, is indicated for combination antiretroviral treatment of HIV-1 infected adults and pediatric patients weighing 36 kg or higher who are treatment-experienced and infected with HIV-1 strains resistant to more than one protease inhibitor (PI)
This indication is based on analyses of plasma HIV-1 RNA levels in two controlled studies of APTIVUS/ritonavir of 48 weeks duration in treatment-experienced adults and one open-label 48-week study in pediatric patients. The adult studies were conducted in clinically advanced, 3-class antiretroviral (NRTI, NNRTI, PI) treatment-experienced adults with evidence of HIV-1 replication despite ongoing antiretroviral therapy.
The following points should be considered when initiating therapy with APTIVUS/ritonavir:
- The use of APTIVUS/ritonavir in treatment-naïve patients is not recommended [
- The use of other active agents with APTIVUS/ritonavir is associated with a greater likelihood of treatment response [
- Genotypic or phenotypic testing and/or treatment history should guide the use of APTIVUS/ritonavir [
- Use caution when prescribing APTIVUS/ritonavir to patients with elevated transaminases, hepatitis B or C co-infection or patients with mild hepatic impairment [
- Liver function tests should be performed at initiation of therapy with APTIVUS/ritonavir and monitored frequently throughout the duration of treatment [
- The drug-drug interaction potential of APTIVUS/ritonavir when co-administered with other drugs must be considered prior to and during APTIVUS/ritonavir use [
- Use caution when prescribing APTIVUS/ritonavir in patients who may be at risk for increased bleeding or who are receiving medications known to increase the risk of bleeding [
There are no study results demonstrating the effect of APTIVUS/ritonavir on clinical progression of HIV-1.
2DOSAGE FORMS AND STRENGTHS
Capsules: 250 mg, pink, oblong capsules imprinted with "TPV 250"
3CONTRAINDICATIONS
- APTIVUS is contraindicated in patients with moderate or severe (Child-Pugh Class B or C, respectively) hepatic impairment [
- APTIVUS/ritonavir is contraindicated when co-administered with drugs that are highly dependent on CYP3A for clearance or are potent CYP3A inducers (see
Due to the need for co-administration of APTIVUS with ritonavir, please refer to the ritonavir prescribing information for a description of ritonavir contraindications.
4ADVERSE REACTIONS
The following adverse reactions are described, in greater detail, in other sections:
- Hepatic Impairment and Toxicity [
- Intracranial Hemorrhage [
- Rash [
Due to the need for co-administration of APTIVUS with ritonavir, please refer to ritonavir prescribing information for ritonavir-associated adverse reactions.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
4.1Clinical Trials in Adults
APTIVUS, co-administered with ritonavir, has been studied in a total of 6308 HIV-1 positive adults as combination therapy in clinical studies. Of these, 1299 treatment-experienced subjects received the dose of 500 mg/200 mg BID. Nine hundred nine (909) adults, including 541 in the 1182.12 and 1182.48 controlled clinical trials, have been treated for at least 48 weeks [
In 1182.12 and 1182.48 in the APTIVUS/ritonavir arm, the most frequent adverse reactions were diarrhea, nausea, pyrexia, vomiting, fatigue, headache, and abdominal pain. The 48-Week Kaplan-Meier rates of adverse reactions leading to discontinuation were 13.3% for APTIVUS/ritonavir-treated subjects and 10.8% for the comparator arm subjects.
Adverse reactions reported in the controlled clinical trials 1182.12 and 1182.48, based on treatment-emergent clinical adverse reactions of moderate to severe intensity (Grades 2 - 4) in at least 2% of treatment-experienced subjects in either treatment group are summarized in Table 2 below.
4.2Clinical Trials in Pediatrics
APTIVUS, co-administered with ritonavir, has been studied in a total of 135 HIV-1 infected pediatric subjects as combination therapy. Study 1182.14 enrolled HIV-1 infected, treatment-experienced pediatric subjects (with the exception of 3 treatment-naïve subjects), with baseline HIV-1 RNA of at least 1500 copies/mL. One hundred and ten (110) subjects were enrolled in a randomized, open-label 48-week clinical trial (Study 1182.14) and 25 subjects were enrolled in other clinical studies including Expanded Access and Emergency Use Programs.
The adverse reactions profile seen in Study 1182.14 was similar to adults. However, rash (5.5%), was reported more frequently in pediatric subjects than in adults.
The most common Grade 3-4 laboratory abnormalities were increases in CPK (11%), ALT (6.5%), and amylase (7.5%).
5OVERDOSAGE
There is no known antidote for APTIVUS overdose. Treatment of overdose should consist of general supportive measures, including monitoring of vital signs and observation of the patient's clinical status. If indicated, elimination of unabsorbed tipranavir should be achieved by emesis or gastric lavage. Administration of activated charcoal may also be used to aid in removal of unabsorbed drug. Since tipranavir is highly protein bound, dialysis is unlikely to provide significant removal of the drug.
6DESCRIPTION
APTIVUS is a protease inhibitor of HIV-1 belonging to the class of 4-hydroxy-5,6-dihydro-2-pyrone sulfonamides.
The chemical name of tipranavir is 2-Pyridinesulfonamide, N-[3-[(1R)-1-[(6R)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2H-pyran-3-yl]propyl]phenyl]-5-(trifluoromethyl). It has a molecular formula of C
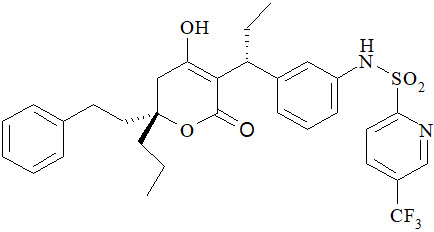
Tipranavir is a white to off-white to slightly yellow solid. It is freely soluble in dehydrated alcohol and propylene glycol, and insoluble in aqueous buffer at pH 7.5.
APTIVUS soft gelatin capsules are for oral administration. Each capsule contains 250 mg tipranavir. The major inactive ingredients in the capsule are dehydrated alcohol (7% w/w or 0.1 g per capsule), polyoxyl 35 castor oil, propylene glycol, mono/diglycerides of caprylic/capric acid and gelatin.
7HOW SUPPLIED/STORAGE AND HANDLING
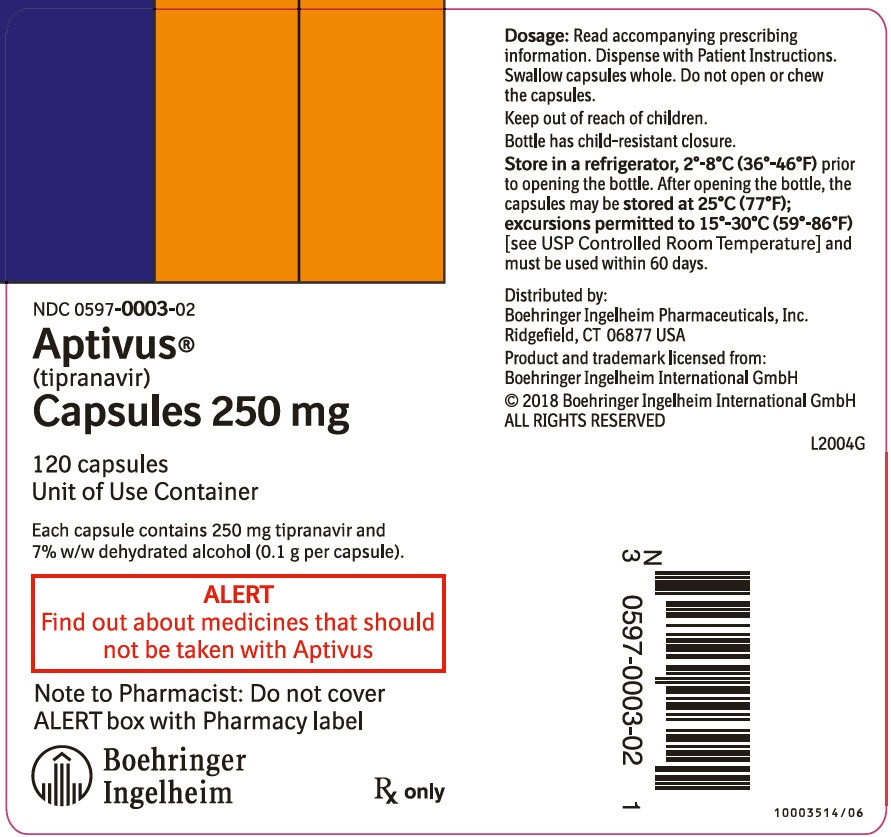
APTIVUS capsules 250 mg are pink, oblong soft gelatin capsules imprinted in black with "TPV 250". They are packaged in HDPE unit-of-use bottles with a child resistant closure and 120 capsules. (NDC 0597-0003-02).
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
9PRINCIPAL DISPLAY PANEL - 250 mg Capsule Bottle Label
NDC 0597-0003-02
Aptivus®
120 capsules
Each capsule contains 250 mg tipranavir and
ALERT
Note to Pharmacist: Do not cover
Boehringer
Rx only