Generic Name
Tobramycin
Brand Names
TOBI, Tobrex, TobraDex, TOBI Podhaler, Bethkis
FDA approval date: June 28, 1981
Classification: Aminoglycoside Antibacterial
Form: Injection, Ointment, Inhalant, Suspension, Capsule, Solution
What is TOBI (Tobramycin)?
Tobramycin Ophthalmic Solution, USP 0.3% is a topical antibiotic indicated in the treatment of external infections of the eye and its adnexa caused by susceptible bacteria. Appropriate monitoring of bacterial response to topical antibiotic therapy should accompany the use of Tobramycin Ophthalmic Solution, USP 0.3%. Clinical studies have shown tobramycin to be safe and effective for use in children.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
TOBI (Tobramycin)
1INDICATIONS AND USAGE
TOBI is indicated for the management of cystic fibrosis in adults and pediatric patients 6 years of age and older with
Safety and efficacy have not been demonstrated in patients under the age of 6 years, patients with forced expiratory volume in 1 second (FEV
2DOSAGE FORMS AND STRENGTHS
TOBI is supplied as a sterile inhalational solution for nebulization in single-dose 5 mL ampoules. Each 5 mL ampoule contains 300 mg of tobramycin.
3CONTRAINDICATIONS
TOBI is contraindicated in patients with a known hypersensitivity to any aminoglycoside.
4ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Bronchospasm
- Ototoxicity
- Nephrotoxicity
- Neuromuscular Disorders
- Embryo-fetal Toxicity
- Concomitant Use of Systemic Aminoglycosides
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
TOBI was studied in two Phase 3 clinical studies involving 258 cystic fibrosis patients ranging in age from 6 to 48 years. Patients received TOBI in alternating periods of 28 days on and 28 days off drug in addition to their standard cystic fibrosis therapy for a total of 24 weeks.
Table 1 lists the percent of patients with selected adverse reactions that occurred in >5% of TOBI patients during the two Phase 3 studies.
Selected adverse reactions that occurred in less than or equal to 5% of patients treated with TOBI:
Ear and Labyrinth Disorders: Tinnitus
Musculoskeletal and Connective Tissue Disorders: Myalgia
Infections and Infestations: Laryngitis
4.1.1Voice Alteration and Tinnitus
Voice alteration and tinnitus were the only adverse reactions reported by significantly more TOBI-treated patients. Thirty-three patients (13%) treated with TOBI complained of voice alteration compared to 17 (7%) placebo patients. Voice alteration was more common in the on-drug periods.
Eight patients from the TOBI group (3%) reported tinnitus compared to no placebo patients. All episodes were transient, resolved without discontinuation of the TOBI treatment regimen, and were not associated with loss of hearing in audiograms. Tinnitus is one of the sentinel symptoms of cochlear toxicity, and patients with this symptom should be carefully monitored for high frequency hearing loss. The numbers of patients reporting vestibular adverse experiences such as dizziness were similar in the TOBI and placebo groups.
4.1.2Changes in Serum Creatinine
Nine (3%) patients in the TOBI group and nine (3%) patients in the placebo group had increases in serum creatinine of at least 50% over baseline. In all nine patients in the TOBI group, creatinine decreased at the next visit.
4.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of TOBI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Ear and Labyrinth Disorders
Hearing loss: Some of these reports occurred in patients with previous or concomitant treatment with systemic aminoglycosides. Patients with hearing loss frequently reported tinnitus
Skin and Subcutaneous Tissue Disorders
Hypersensitivity, pruritus, urticaria, rash
Nervous System Disorders
Aphonia, dysgeusia
Respiratory, Thoracic, and Mediastinal Disorders
Bronchospasm
Metabolism and Nutrition Disorders
Decreased appetite
5OVERDOSAGE
Signs and symptoms of acute toxicity from overdosage of intravenous (IV) tobramycin might include dizziness, tinnitus, vertigo, loss of high-tone hearing acuity, respiratory failure, neuromuscular blockade, and renal impairment. Administration by inhalation results in low systemic bioavailability of tobramycin. Tobramycin is not significantly absorbed following oral administration. Tobramycin serum concentrations may be helpful in monitoring overdosage.
Acute toxicity should be treated with immediate withdrawal of TOBI, and baseline tests of renal function should be undertaken.
In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment. In the case of any overdosage, the possibility of drug interactions with alterations in drug disposition should be considered.
Hemodialysis may be helpful in removing tobramycin from the body.
6DESCRIPTION
TOBI
Each single-dose 5 mL ampoule contains 300 mg tobramycin and 11.25 mg sodium chloride in sterile water for injection. Sulfuric acid and sodium hydroxide are added to adjust the pH to 6.0. Nitrogen is used for sparging. All ingredients meet USP requirements. The formulation contains no preservatives.
7CLINICAL STUDIES
Two identically designed, double-blind, randomized, placebo-controlled, parallel group, 24-week clinical studies (Study 1 and Study 2) at a total of 69 cystic fibrosis centers in the United States were conducted in cystic fibrosis patients with
All subjects had baseline FEV
All patients received either TOBI or placebo (saline with 1.25 mg quinine for flavoring) in addition to standard treatment recommended for cystic fibrosis patients, which included oral and parenteral antipseudomonal therapy, β2-agonists, cromolyn, inhaled steroids, and airway clearance techniques. In addition, approximately 77% of patients were concurrently treated with dornase alfa (PULMOZYME, Genentech).
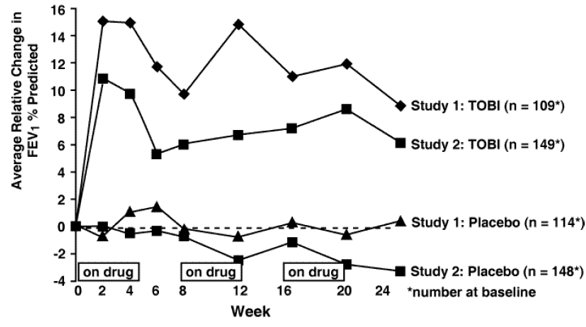
In each study, TOBI-treated patients experienced significant improvement in pulmonary function. Improvement was demonstrated in the TOBI group in Study 1 by an average increase in FEV
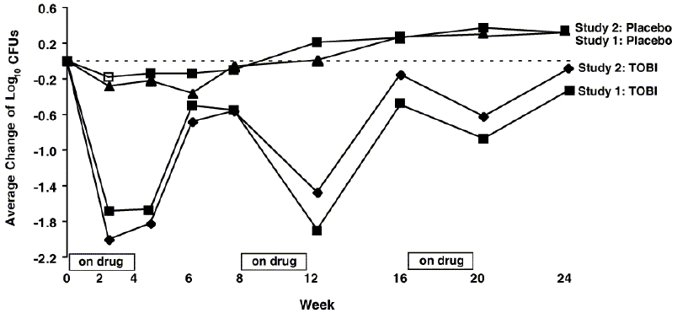
In each study, TOBI therapy resulted in a significant reduction in the number of
Patients treated with TOBI were hospitalized for an average of 5.1 days compared to 8.1 days for placebo patients. Patients treated with TOBI required an average of 9.6 days of parenteral antipseudomonal, antibacterial treatment compared to 14.1 days for placebo patients. During the 6 months of treatment, 40% of TOBI patients and 53% of placebo patients were treated with parenteral antipseudomonal antibacterials.
The relationship between
Treatment with TOBI did not affect the susceptibility of the majority of
8REFERENCES
- Neu HC. Tobramycin: an overview. [Review]. J Infect Dis 1976; Suppl 134:S3-19.
- Weber A, Smith A, Williams-Warren J et al. Nebulizer delivery of tobramycin to the lower respiratory tract. Pediatr Pulmonol 1994; 17 (5):331-9.
- Bryan LE. Aminoglycoside resistance. Bryan LE, Ed. Antimicrobial drug resistance. Orlando, FL: Academic Press, 1984: 241-77.
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (
Difficulty Breathing:
Advise patients to inform their physicians if they experience shortness of breath or wheezing after administration of tobramycin inhalation solution. Tobramycin inhalation solution can cause a narrowing of the airway
Hearing Loss:
Advise patients to inform their physician if they experience ringing in the ears, dizziness, or any changes in hearing because tobramycin inhalation solution has been associated with hearing loss
Kidney Damage:
Advise patients to inform their physician if they have any history of kidney problems because tobramycin inhalation solution is in a class of drugs that have caused kidney damage
Embryo-fetal Toxicity:
Advise pregnant women that aminoglycosides can cause irreversible congenital deafness when administered to a pregnant woman
Lactation:
Advise a woman to monitor their breastfed infants for diarrhea and/or bloody stools
Manufactured for:
Manufactured by:
© 2023 Viatris Inc.
TOBI is a registered trademark of BGP Products Operations GmbH, a Viatris Company.
The brands listed are trademarks of their respective owners.
WS:TOBRIS:R2
10PATIENT INFORMATION
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 2/2023
© 2023 Viatris Inc.
TOBI is a registered trademark of BGP Products Operations GmbH, a Viatris Company.
The brands listed are trademarks of their respective owners.
WS:PIL:TOBRIS:R2
11Instructions for Use
TOBI (TOH-bee)
Read this Instructions for Use before you start using TOBI inhalation solution and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
TOBI is made for inhalation using a PARI LC PLUS™ Reusable Nebulizer and a DeVilbiss® Pulmo-Aide® air compressor. TOBI can be taken at home, school, or at work. The following instructions tell you how to use the DeVilbiss Pulmo-Aide air compressor and PARI LC PLUS Reusable Nebulizer to administer TOBI.
You will need the following supplies (See Figure A):
- 1 TOBI plastic ampule (TOBI is packaged with 4 ampules in each foil pouch)
- DeVilbiss Pulmo-Aide air compressor
- PARI LC PLUS Reusable Nebulizer
- Tubing to connect the nebulizer and compressor
- Clean paper or cloth towels
- Nose clips (optional)
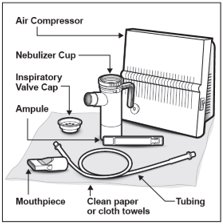
It is important that your nebulizer and compressor function properly before starting your TOBI therapy.
Note: Read the manufacturer care and use instructions for important information.
Prepare Your TOBI for Inhalation Therapy
Step 1: Wash your hands thoroughly with soap and water.
Step 2: Open the foil pouch.
Step 3: Separate 1 TOBI ampule by gently pulling apart at the bottom tabs (See Figure B). Place the remaining TOBI ampules in the refrigerator.
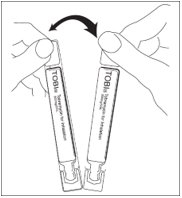
Step 4: Check the expiration date stamped on the TOBI ampule (See Figure C). Do not use the TOBI ampule if the expiration date has passed.

Step 5: Check that the TOBI ampule medicine is clear and does not have particles.
- Unrefrigerated TOBI, which is normally slightly yellow, may darken with age. This color change does not mean there is any change in the quality of the medicine.
- Do not use the TOBI ampule if the medicine is cloudy or has particles.
- Throw it away and get a new one.
Step 6: Lay out the parts of a PARI LC PLUS Reusable Nebulizer package on a clean, dry paper or cloth towel. You should have the following parts (See Figure D):
- Nebulizer Top and Bottom (Nebulizer Cup) Assembly
- Inspiratory Valve Cap
- Mouthpiece with Valve
- Tubing
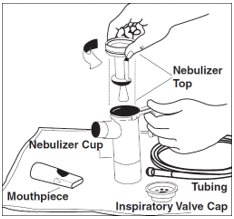
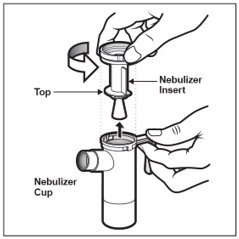
Step 7: Remove the Nebulizer Top from the Nebulizer Cup by twisting the Nebulizer Top counter-clockwise, and then lifting off (See Figure E).
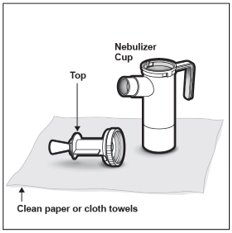
Step 8: Place the Nebulizer Top on the clean paper or cloth towel by standing the Nebulizer Cup upright on the towel (See Figure F).
Step 9: Connect one end of the tubing to the compressor air outlet (See Figure G). The tubing should fit tightly.
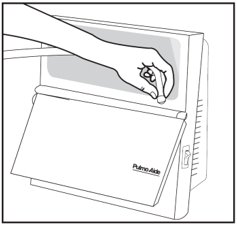
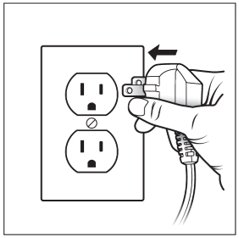
Step 10: Plug in your compressor to an electrical outlet (See Figure H).
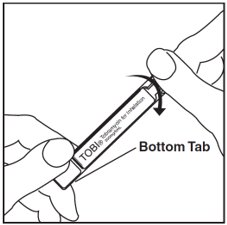
Step 11: Open the TOBI ampule by holding the bottom tab with 1 hand and twisting off the top of the TOBI ampule with the other hand (See Figure I). Be careful not to squeeze the TOBI ampule until you are ready to empty all the medicine into the Nebulizer Cup.
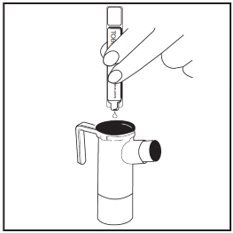
Step 12: Squeeze all the medicine of the TOBI ampule into the Nebulizer Cup (See Figure J).
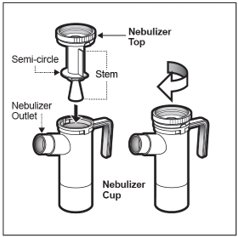
Step 13: Replace the Nebulizer Top. To replace the Nebulizer Top insert the Nebulizer Top into the Nebulizer Cup with the semi-circle halfway down the stem of the Nebulizer Top facing the Nebulizer Outlet. Turn the Nebulizer Top clockwise until securely fastened to the nebulizer Cup. (See Figure K).
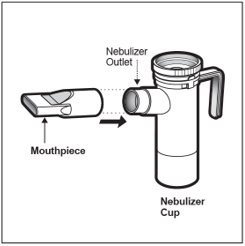
Step 14: Push the Mouthpiece straight onto the Nebulizer Outlet (See Figure L).
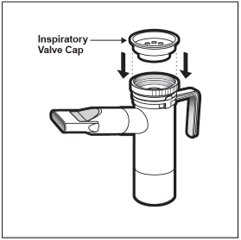
Step 15: Firmly push the Inspiratory Valve Cap straight down onto the Nebulizer Top (See Figure M). The Inspiratory Valve Cap will fit tightly.
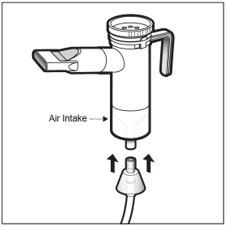
Step 16: Hold the Nebulizer Cup upright and firmly push the free end of the tubing from the compressor to the Air Intake on the bottom of the Nebulizer Cup (See Figure N).Make sure to keep the Nebulizer Cup upright.
Giving your TOBI Inhalation Therapy
Step 17: Turn on the compressor (See Figure O).
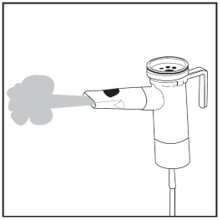
Step 18: Check for a steady mist from the Mouthpiece (See Figure P). If there is no mist, check all tubing connections and make sure that the compressor is working properly.
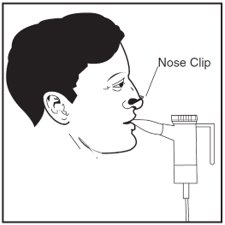
Step 19: Sit or stand in an upright position that will allow you to breathe normally. Place the Mouthpiece between your teeth and on top of your tongue and breathe normally only through your mouth (See Figure Q). Nose clips may help you breathe through your mouth and not through your nose. Do not block the airflow with your tongue.
Step 20: Keep breathing in your TOBI medicine for at least 15 minutes to get your full dose. Continue therapy until all your TOBI medicine is gone, and there is no longer any mist being made. You may hear a sputtering sound coming from the Mouthpiece when the Nebulizer Cup is empty. The entire TOBI therapy should take about 15 minutes to complete.
If you are interrupted, need to cough or rest during your TOBI treatment, turn off the compressor to save your medicine. Turn the compressor back on when you are ready to restart your treatment.
Follow the nebulizer cleaning and disinfecting instructions after completing your therapy.
After your TOBI Inhalation Therapy
Cleaning Your Nebulizer
To reduce the risk of infection, illness or injury from contamination, you must thoroughly clean all parts of the nebulizer as instructed after each treatment. Never use a nebulizer with a clogged nozzle. If the nozzle is clogged, no aerosol mist is made, and your therapy will not be as effective. Replace the nebulizer if clogging occurs.
1) Remove tubing from nebulizer and disassemble nebulizer parts.
2) Wash all parts (except tubing) with warm water and liquid dish soap.
3) Rinse thoroughly with warm water and shake out water.
4) Air dry or hand dry nebulizer parts on a clean, lint-free cloth. Reassemble nebulizer when dry, and store.
You can also wash all parts of the nebulizer in a dishwasher (except tubing).
1) Place the nebulizer parts in a dishwasher basket.
2) Place the dishwasher basket on the top rack of the dishwasher.
3) Remove and dry the parts when the cycle is complete.
Disinfecting Your Nebulizer
Your nebulizer is for your use only.
Clean the nebulizer as described above. Every other treatment day, disinfect the nebulizer parts (except tubing) by boiling them in water for a full 10 minutes. Dry parts on a clean, lint-free cloth.
Care and Use of Your Pulmo-Aide Compressor
Follow the manufacturer instructions for care and use of your compressor.
Filter Change:
- DeVilbiss Compressor filters should be changed every 6 months or sooner if the filter turns completely gray in color.
Compressor Cleaning:
- With power switch in the “Off” position, unplug power cord from wall outlet.
- Wipe outside of the compressor cabinet with a clean, damp cloth every few days to keep dust free.
Caution:
How should I store TOBI?
- Store TOBI ampules in a refrigerator between 36°F to 46°F (2°C to 8°C) until needed.
- You may store the TOBI ampules in the foil pouches (opened or unopened) at room temperature 77°F (25°C) for up to 28 days.
- Do not use TOBI ampules if they have been stored at room temperature for more than 28 days.
- Protect TOBI ampules from light.
Keep TOBI and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Additional Information
Nebulizer: 1-800-327-8632
Compressor: 1-800-338-1988
TOBI: Call Mylan at 1-877-446-3679 (1-877-4-INFO-RX).
Manufactured for:
Manufactured by:
© 2023 Viatris Inc.
TOBI is a registered trademark of BGP Products Operations GmbH, a Viatris Company.
The brands listed are trademarks of their respective owners.
WS:IFU:TOBRIS:R2
Revised: 2/2023
12PRINCIPAL DISPLAY PANEL – 300 mg / 5 mL Ampules
NDC 49502-345-73
TOBI
300 mg / 5 mL Ampules
Rx only
Store In Refrigerator
Contents: Each foil pouch contains four sterile, non-pyrogenic, single-use ampules. Each 5 mL ampule contains one 300 mg dose of Tobramycin, USP and 11.25 mg Sodium Chloride in Sterile Water for Injection.
Dosage and Administration: Each single-use ampule contains one 300 mg dose.
For inhalation only using required type of nebulizer. See package insert for full prescribing information.
Storage: Store in a refrigerator at 2-8°C/36-46°F. Protect from intense light. See package insert for additional information.
For more information, call
Manufactured for:
Manufactured by:
© 2021 Viatris Inc.
TOBI is a registered trademark of BGP Products Operations GmbH, a Viatris Company.
WS:345:56C:R1
PCR-700-13894
