Brand Name
Zemdri
Generic Name
Plazomicin
View Brand Information FDA approval date: June 29, 2018
Form: Injection
What is Zemdri (Plazomicin)?
ZEMDRI is an aminoglycoside antibacterial indicated for the treatment of patients 18 years of age or older with Complicated Urinary Tract Infections including Pyelonephritis.
Approved To Treat
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Zemdri (plazomicin) (Plazomicin)
WARNING: NEPHROTOXICITY, OTOTOXICITY, NEUROMUSCULAR BLOCKADE and FETAL HARM
- Nephrotoxicity has been reported with ZEMDRI. The risk of nephrotoxicity is greater in patients with impaired renal function, the elderly, and in those receiving concomitant nephrotoxic medications. Assess creatinine clearance in all patients prior to initiating therapy and daily during therapy [see . Therapeutic Drug Monitoring (TDM) is recommended for complicated urinary tract infection (cUTI) patients with CLcr less than 90 mL/min to avoid potentially toxic levels [see .
- Ototoxicity, manifested as hearing loss, tinnitus, and/or vertigo, has been reported with ZEMDRI. Symptoms of aminoglycoside-associated ototoxicity may be irreversible and may not become evident until after completion of therapy. Aminoglycoside-associated ototoxicity has been observed primarily in patients with a family history of hearing loss, patients with renal impairment, and in patients receiving higher doses and/or longer durations of therapy than recommended
- Aminoglycosides have been associated with neuromuscular blockade. During therapy with ZEMDRI, monitor for adverse reactions associated with neuromuscular blockade, particularly in high-risk patients, such as patients with underlying neuromuscular disorders (including myasthenia gravis) or in patients concomitantly receiving neuromuscular blocking agents [see .
- Aminoglycosides, including ZEMDRI, can cause fetal harm when administered to a pregnant woman [see .
1DOSAGE FORMS AND STRENGTHS
ZEMDRI injection 500 mg/10 mL (50 mg/mL) is a sterile, clear, colorless to yellow solution supplied in a single-dose vial. Each single-dose vial contains plazomicin sulfate equivalent to 500 mg plazomicin freebase.
2CONTRAINDICATIONS
ZEMDRI is contraindicated in patients with known hypersensitivity to any aminoglycoside
3ADVERSE REACTIONS
The following important adverse reactions are discussed in greater detail in the Warnings and Precautions section:
- Nephrotoxicity
- Ototoxicity
- Neuromuscular Blockade
- Fetal Harm
- Hypersensitivity Reactions
- Clostridium difficile-Associated Diarrhea [see
3.1Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be compared directly to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
ZEMDRI was evaluated in two comparator-controlled clinical trials (Trial 1, NCT02486627 and Trial 2, NCT01096849) in patients with cUTI, including pyelonephritis. In both trials, patients with CLcr greater than 60 mL/min received ZEMDRI 15 mg/kg IV once daily as a 30-minute infusion
Trial 1 included 303 patients treated with ZEMDRI and 301 patients treated with meropenem. Patients were to receive 4 to 7 days of ZEMDRI (mean duration of 5.1 days). In some patients, parenteral therapy was followed by a switch to an oral antibacterial drug.
The median age of patients treated with ZEMDRI in Trial 1 was 62 years (range 18 to 90 years) and 45.2% of patients were 65 years of age or older. Patients treated with ZEMDRI were predominantly female (56.1%) and White (99.3%). A majority of patients (68.0%) had mild or moderate renal impairment (CLcr >30 to 90 mL/min) at baseline. Patients with CLcr of 30 mL/min or less were excluded.
4OVERDOSAGE
In the event of overdosage, ZEMDRI should be discontinued and supportive care is advised. Maintenance of glomerular filtration and careful monitoring of renal function is recommended. Hemodialysis may aid in the removal of ZEMDRI from the blood, especially if renal function is, or becomes, compromised. No clinical information is available on the use of hemodialysis to treat ZEMDRI overdosage.
5DESCRIPTION
ZEMDRI contains plazomicin sulfate, a semi-synthetic aminoglycoside antibacterial derived from sisomicin. The chemical name of plazomicin sulfate is (2"
Figure 1: Chemical Structure of Plazomicin Sulfate
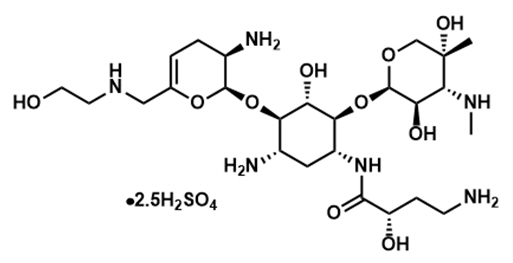
ZEMDRI injection 500 mg/10 mL is a sterile, clear, colorless-to-yellow liquid for intravenous administration supplied in 10-mL single-dose Type 1 glass vials. Each vial contains plazomicin sulfate equivalent to 500 mg plazomicin freebase at a concentration of 50 mg/mL adjusted to pH 6.5. Each vial also contains Water for Injection and sodium hydroxide for pH adjustment. This sterile, nonpyrogenic solution is formulated without preservatives.
6REFERENCES
- American Speech-Language-Hearing Association. (1994). Audiologic management of individuals receiving cochleotoxic drug therapy [Guidelines]. Available from www.asha.org/policy.
7PRINCIPAL DISPLAY PANEL - 10 mL Vial Carton
NDC 71045-010-02
ZEMDRI™
10 (10 mL) Single-dose vials
For Intravenous Infusion Only





