Brand Name
Tykerb
Generic Name
Lapatinib
View Brand Information FDA approval date: August 03, 2016
Classification: Kinase Inhibitor
Form: Tablet
What is Tykerb (Lapatinib)?
TYKERB ® is indicated in combination with: capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress human epidermal growth factor receptor 2 and who have received prior therapy including an anthracycline, a taxane, and trastuzumab. Limitations of Use : Patients should have disease progression on trastuzumab prior to initiation of treatment with TYKERB in combination with capecitabine. letrozole for the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer that overexpresses the HER2 receptor for whom hormonal therapy is indicated. TYKERB in combination with an aromatase inhibitor has not been compared to a trastuzumab-containing chemotherapy regimen for the treatment of metastatic breast cancer. TYKERB is a kinase inhibitor indicated in combination with: capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress human epidermal growth factor receptor 2 and who have received prior therapy, including an anthracycline, a taxane, and trastuzumab. Limitations of Use : Patients should have disease progression on trastuzumab prior to initiation of treatment with TYKERB in combination with capecitabine. letrozole for the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer that overexpresses the HER2 receptor for whom hormonal therapy is indicated. TYKERB in combination with an aromatase inhibitor has not been compared to a trastuzumab-containing chemotherapy regimen for the treatment of metastatic breast cancer.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
TYKERB (lapatinib)
WARNING: HEPATOTOXICITY
Hepatotoxicity has been observed in clinical trials and postmarketing experience. The hepatotoxicity may be severe and deaths have been reported. Causality of the deaths is uncertain
1INDICATIONS AND USAGE
TYKERB
- capecitabine for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress human epidermal growth factor receptor 2 (HER2) and who have received prior therapy, including an anthracycline, a taxane, and trastuzumab.
- letrozole for the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer that overexpresses the HER2 receptor for whom hormonal therapy is indicated.
TYKERB in combination with an aromatase inhibitor has not been compared to a trastuzumab-containing chemotherapy regimen for the treatment of metastatic breast cancer.
2DOSAGE FORMS AND STRENGTHS
250 mg tablets — oval, biconvex, orange, film-coated with ‘GS XJG’ debossed on one side.
3CONTRAINDICATIONS
TYKERB is contraindicated in patients with known severe hypersensitivity (e.g., anaphylaxis) to this product or any of its components.
4OVERDOSAGE
There is no known antidote for overdoses of TYKERB. The maximum oral doses of lapatinib that have been administered in clinical trials are 1,800 mg once daily. More frequent ingestion of TYKERB could result in serum concentrations exceeding those observed in clinical trials and could result in increased toxicity. Therefore, missed doses should not be replaced and dosing should resume with the next scheduled daily dose.
Asymptomatic and symptomatic cases of overdose have been reported. The doses ranged from 2,500 to 9,000 mg daily and where reported, the duration varied between 1 and 17 days. Symptoms observed include TYKERB-associated events
Because TYKERB is not significantly renally excreted and is highly bound to plasma proteins, hemodialysis would not be expected to be an effective method to enhance the elimination of lapatinib.
Treatment of overdose with TYKERB should consist of general supportive measures.
5DESCRIPTION
Lapatinib is a small molecule and a member of the 4-anilinoquinazoline class of kinase inhibitors. It is present as the monohydrate of the ditosylate salt, with chemical name
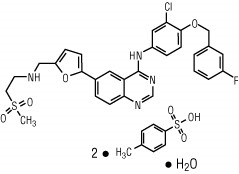
Lapatinib is a yellow solid, and its solubility in water is 0.007 mg/mL and in 0.1N HCl is 0.001 mg/mL at 25°C.
Each 250 mg tablet of TYKERB contains 405 mg of lapatinib ditosylate monohydrate, equivalent to 398 mg of lapatinib ditosylate or 250 mg lapatinib free base.
The inactive ingredients of TYKERB are:
6HOW SUPPLIED/STORAGE AND HANDLING
The 250 mg tablets of TYKERB are oval, biconvex, orange, and film-coated with ‘GS XJG’ debossed on one side and are available in:
Bottles of 150 tablets: NDC 0078-0671-19
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
7PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform patients of the following:
Decreased Left Ventricular Ejection Fraction (LVEF)
- TYKERB has been reported to decrease left ventricular ejection fraction which may result in shortness of breath, palpitations, and/or fatigue
Hepatotoxicity and Hepatic Impairment
- Periodic laboratory testing will be performed while taking TYKERB. Advise patients to report signs and symptoms of liver dysfunction to their healthcare provider right away
Diarrhea
- TYKERB often causes diarrhea which may be severe in some cases
Interstitial Lung Disease/Pneumonitis
- Advise patients to report pulmonary signs or symptoms indicative of ILD or pneumonitis
Severe Cutaneous Reactions
- Advise patients to report severe cutaneous reactions to their healthcare provider if they develop these symptoms while taking TYKERB
Drug and Food Interactions
- TYKERB may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products
- TYKERB may interact with grapefruit. Advise patients not to take TYKERB with grapefruit products
Dosing Administration
- TYKERB should be taken at least one hour before or one hour after a meal, in contrast to capecitabine which should be taken with food or within 30 minutes after food. The dose of TYKERB should be taken once daily. Dividing the daily dose is not recommended
Embryo-Fetal Toxicity
- Inform female patients of the risk to a fetus and potential loss of the pregnancy. Advise females to inform their healthcare provider if they are pregnant or become pregnant
- Advise females of reproductive potential to use effective contraception during treatment with TYKERB and for 1 week after the last dose.
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 1 week following the last dose
Lactation
- Advise patients not to breastfeed during treatment and for 1 week after the last dose of TYKERB
Distributed by
© Novartis
T2022-23
8PRINCIPAL DISPLAY PANEL
NDC 0078-0671-19
NOVARTIS
Tykerb
250 mg
150 Tablets
Rx only
