Generic Name
BuPROPion
Brand Names
Auvelity, Wellbutrin, Aplenzin
FDA approval date: November 15, 1996
Classification: Aminoketone
Form: Tablet
What is Auvelity (BuPROPion)?
Bupropion hydrochloride extended-release tablets is indicated for the treatment of major depressive disorder , as defined by the Diagnostic and Statistical Manual . The efficacy of bupropion in the treatment of a major depressive episode was established in two 4-week controlled inpatient trials and one 6-week controlled outpatient trial of adult subjects with MDD. The efficacy of bupropion hydrochloride extended-release tablets in maintaining an antidepressant response for up to 44 weeks following 8 weeks of acute treatment was demonstrated in a placebo-controlled trial.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
AUVELITY (dextromethorphan hydrobromide, bupropion hydrochloride)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and emergence of suicidal thoughts and behaviors
1INDICATIONS AND USAGE
AUVELITY is indicated for the treatment of major depressive disorder (MDD) in adults.
2DOSAGE FORMS AND STRENGTHS
AUVELITY extended-release tablets contain 45 mg dextromethorphan hydrobromide and 105 mg bupropion hydrochloride. The tablets are beige and round with “45/105” debossed on one side.
3CONTRAINDICATIONS
AUVELITY is contraindicated in patients:
- with a seizure disorder
- with a current or prior diagnosis of bulimia or anorexia nervosa as a higher incidence of seizures was observed in such patients treated with the immediate-release formulation of bupropion
- undergoing abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiepileptic drugs
- taking, or within 14 days of stopping, MAOIs due to the risk of serious and possibly fatal drug interactions, including hypertensive crisis and serotonin syndrome
- with known hypersensitivity to bupropion, dextromethorphan, or other components of AUVELITY. Anaphylactoid/anaphylactic reactions and Stevens-Johnson syndrome have been reported with bupropion. Arthralgia, myalgia, fever with rash, and other serum sickness-like symptoms suggestive of delayed hypersensitivity have also been reported with bupropion
4ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults
- Seizure
- Increased Blood Pressure and Hypertension
- Activation of Mania or Hypomania
- Psychosis and Other Neuropsychiatric Reactions
- Angle-closure Glaucoma
- Dizziness
- Serotonin Syndrome
- Embryo-fetal Toxicity
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
AUVELITY was evaluated for safety in a total of 1114 patients with MDD or another indication from four studies (two 6-week studies in MDD, one 6-week study in another indication, and one long-term study in MDD and another indication). One 6-week study in MDD employed placebo as a control arm. Two 6-week studies, one in MDD and one in another indication, employed bupropion as a control arm. In the patients treated with AUVELITY in the long-term study (n=876), 597 received at least 6 months of treatment, and 110 received at least 12 months of treatment.
The data below are based on the 6-week, placebo-controlled study in which either AUVELITY (n=162) or placebo (n=164) was administered twice daily to patients with MDD (Study 1). Demographics of the patients who participated in this study are summarized in Clinical Studies
Adverse Reactions Leading to Discontinuation
In the 6-week placebo-controlled study, 4% of patients treated with AUVELITY and 0% of placebo-treated patients discontinued participation due to adverse reactions. The adverse reaction that led to study discontinuation in ≥1% of patients treated with AUVELITY was anxiety (2%).
Most Common Adverse Reactions
In the 6-week placebo-controlled clinical study, the most common (incidence ≥5% for AUVELITY and more than twice as frequently as placebo) adverse reactions were dizziness (16%), headache (8%), diarrhea (7%), somnolence (7%), dry mouth (6%), sexual dysfunction (6%), and hyperhidrosis (5%).
Table 2 shows the incidence of adverse reactions that occurred in ≥2% of patients treated with AUVELITY and more frequently than in patients treated with placebo in Study 1.
4.2Postmarketing Experience
The following adverse reactions have been identified with the use of the individual components of AUVELITY, dextromethorphan and bupropion, during postmarketing use. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dextromethorphan
Drowsiness, dizziness, nervousness or restlessness, nausea, vomiting, and stomach pain.
Bupropion
Body (General): Arthralgia, myalgia, and fever with rash and other symptoms suggestive of delayed hypersensitivity. These symptoms may resemble serum sickness.
Cardiovascular: Complete atrioventricular block, extrasystoles, hypotension, hypertension (in some cases severe), phlebitis, pulmonary embolism, and Brugada pattern/syndrome.
Digestive: Colitis, esophagitis, gastrointestinal hemorrhage, gum hemorrhage, hepatitis, intestinal perforation, pancreatitis, and stomach ulcer.
Endocrine: Hyperglycemia, hypoglycemia, hyponatremia, and syndrome of inappropriate antidiuretic hormone secretion.
Hemic and Lymphatic: Anemia, leukocytosis, leukopenia, lymphadenopathy, pancytopenia, and thrombocytopenia. Altered PT and/or INR, infrequently associated with hemorrhagic or thrombotic complications, were observed when bupropion was coadministered with warfarin.
Metabolic and Nutritional: Glycosuria.
Musculoskeletal: Muscle rigidity/fever/rhabdomyolysis and muscle weakness.
Nervous System: Abnormal electroencephalogram (EEG), aggression, agitation, akinesia, aphasia, coma, completed suicide, delirium, delusions, depression, dysarthria, euphoria, extrapyramidal syndrome (dyskinesia, dystonia, hypokinesia, parkinsonism), hallucinations, homicidal ideation, hostility, increased libido, manic reaction, neuralgia, neuropathy, panic, paranoid ideation, psychosis, restlessness, suicide ideation, suicide attempt, unmasking tardive dyskinesia, and aseptic meningitis.
Respiratory: Pneumonia.
Skin and subcutaneous tissue disorders: Alopecia, angioedema, exfoliative dermatitis, hirsutism, Stevens-Johnson syndrome, acute generalized exanthematous pustulosis, and drug reaction with eosinophilia and systemic symptoms (DRESS).
Special Senses: Deafness, increased intraocular pressure, and mydriasis.
Urogenital: Abnormal ejaculation, cystitis, dyspareunia, dysuria, gynecomastia, menopause, painful erection, salpingitis, urinary incontinence, urinary retention, and vaginitis.
5OVERDOSAGE
Human Experience
There is limited clinical study experience regarding human overdosage with AUVELITY. Overdosage information is based on experience with the individual components, dextromethorphan and bupropion. Metabolism of the dextromethorphan component of AUVELITY is inhibited by the bupropion component, such that overdose due to AUVELITY might be more severe or more persistent compared to overdose of dextromethorphan alone.
Dextromethorphan
Symptoms of dextromethorphan overdose include nausea, vomiting, stupor, coma, respiratory depression, seizures, tachycardia, hyperexcitability, and toxic psychosis. Other adverse effects include ataxia, nystagmus, dystonia, blurred vision, and changes in muscle reflexes. Dextromethorphan may cause serotonin syndrome, and this risk is increased by overdose, particularly if taken with other serotonergic agents, SSRIs or tricyclic antidepressants.
Bupropion
Overdoses of up to 30 grams or more of bupropion (approximately 143 times the maximum recommended dose of AUVELITY) have been reported. Seizure was reported in approximately one-third of all cases. Other serious reactions reported with overdoses of bupropion alone included hallucinations, loss of consciousness, mental status changes, sinus tachycardia, ECG changes such as conduction disturbances (including QRS prolongation) or arrhythmias, clonus, myoclonus, and hyperreflexia. Fever, muscle rigidity, rhabdomyolysis, hypotension, stupor, coma, and respiratory failure have been reported mainly when bupropion was part of multiple drug overdoses.
Although most patients recovered without sequelae, deaths associated with overdoses of bupropion alone have been reported in patients ingesting large doses of the drug. Multiple uncontrolled seizures, bradycardia, cardiac failure, and cardiac arrest prior to death were reported in these patients.
Overdosage Management
Treatment of dextromethorphan overdosage should be directed at symptomatic and supportive measures.
There are no known antidotes for bupropion. In case of an overdose, provide supportive care, including close medical supervision and monitoring. Consider the possibility of multiple drug overdose. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. Induction of emesis is not recommended.
Consider contacting a Poison Center (1-800-222-1222) or a medical toxicologist for overdosage management recommendations for AUVELITY.
6DESCRIPTION
AUVELITY is a combination of dextromethorphan hydrobromide, an uncompetitive NMDA receptor antagonist and sigma-1 receptor agonist, and bupropion hydrochloride, an aminoketone and CYP450 2D6 inhibitor.
The chemical name of dextromethorphan hydrobromide is morphinan, 3- methoxy-17-methyl-, (9α, 13α, 14α), hydrobromide monohydrate. Dextromethorphan hydrobromide has the empirical formula C
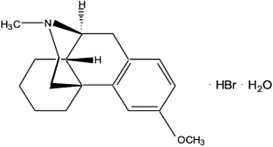
Dextromethorphan hydrobromide powder is white or almost white, crystalline, and sparingly soluble in water.
The chemical name of bupropion hydrochloride is:(±)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1propanone hydrochloride. Bupropion hydrochloride has the empirical formula C
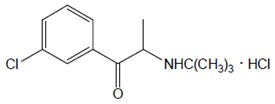
Bupropion hydrochloride powder is white and highly soluble in water.
AUVELITY is for oral administration and is available as round bilayer tablets. Each tablet contains 45 mg dextromethorphan hydrobromide (equivalent to 32.98 mg dextromethorphan base) in an immediate-release formulation and 105 mg bupropion hydrochloride (equivalent to 91.14 mg bupropion base) in an extended-release formulation. Each tablet contains the following inactive ingredients: carbomer homopolymer, colloidal silicon dioxide, crospovidone, glyceryl monocaprylocaprate, l-cysteine hydrochloride monohydrate, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol, red iron oxide, sodium lauryl sulfate, stearic acid, talc, titanium dioxide, and yellow iron oxide.
7CLINICAL STUDIES
The efficacy of AUVELITY for the treatment of MDD in adults was demonstrated in a placebo-controlled clinical study (Study 1,
In Study 1, adult patients (18 to 65 years of age) who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for MDD were randomized to receive AUVELITY (45 mg of dextromethorphan hydrobromide and 105 mg of bupropion hydrochloride) twice daily (N=156) or placebo twice daily (N=162) for 6 weeks. Patients in Study 1 had a median age of 41 years and were 67% female, 55% Caucasian, 35% Black, and 5% Asian.
The primary outcome measure was the change from baseline to Week 6 in the total score of the Montgomery-Asberg Depression Rating Scale (MADRS). The MADRS is a clinician-rated scale used to assess the severity of depressive symptoms. Patients are rated on 10 items to assess feelings of sadness, inner tension, reduced sleep or appetite, difficulty concentrating, lassitude, lack of interest, pessimism, and suicidality. Scores on the MADRS range from 0 to 60, with higher scores indicating more severe depression. AUVELITY was statistically significantly superior to placebo in improvement of depressive symptoms as measured by decrease in MADRS total score at Week 6 (see
The change from baseline in MADRS total score by week in Study 1 is displayed in Figure 3. The change in MADRS total score from baseline to Week 1 and from baseline to Week 2 were pre-specified secondary efficacy endpoints. The difference between AUVELITY and placebo in change from baseline in MADRS total score was statistically significant at Week 1 and at Week 2.
Figure 3: Change from Baseline in MADRS Total Score by Week (Study 1)
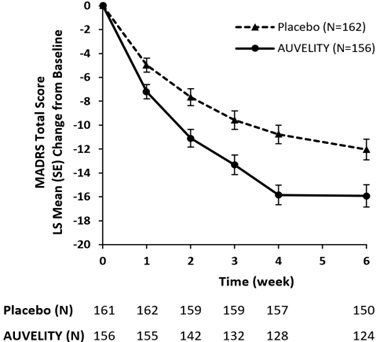
SE = Standard Error
Examination of demographic subgroups by age, sex, and race did not suggest differences in response.
In Study 2, patients with MDD were randomized to receive AUVELITY or bupropion hydrochloride sustained-release tablets 105 mg twice daily for 6 weeks. The primary outcome measure was calculated by assessing the change from baseline in total MADRS score at each on-site visit from Week 1 to Week 6 and then taking the average of those scores. The results of the study demonstrated that dextromethorphan contributes to the antidepressant properties of AUVELITY.
8HOW SUPPLIED/STORAGE AND HANDLING
AUVELITY (dextromethorphan hydrobromide and bupropion hydrochloride) extended-release tablets are beige, film-coated, round, bilayer tablets with “45/105” debossed on one side. AUVELITY is supplied in the following package configuration:
- Dextromethorphan hydrobromide 45mg/bupropion hydrochloride 105 mg:
- Dextromethorphan hydrobromide 45mg/bupropion hydrochloride 105 mg:
Store AUVELITY in original bottle at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
9PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidal ideation and behavior, especially early during treatment and when the dose is adjusted up or down, and instruct them to report such symptoms to the healthcare provider
Hypersensitivity
Advise patients that both immediate and delayed hypersensitivity reactions to AUVELITY could occur. Instruct patients to seek medical attention immediately if they experience symptoms indicative of hypersensitivity, such as skin rash, pruritus, hives, chest pain, edema, shortness of breath, arthralgia, myalgia, or fever after taking AUVELITY
Seizure
Advise patients that AUVELITY can cause seizure and that excessive use or abrupt discontinuation of alcohol, benzodiazepines, antiepileptic drugs, or sedatives/hypnotics can increase the risk. Advise patients to minimize or avoid use of alcohol. Instruct patients to use AUVELITY as directed and to discontinue, and not restart, AUVELITY if they experience a seizure while on treatment
Increased Blood Pressure and Hypertension
Advise patients that AUVELITY can cause increased blood pressure and hypertension and that the risk is increased if used with some other medications such as MAOIs and drugs that increase dopaminergic or noradrenergic activity
Activation of Mania or Hypomania
Advise patients to observe for signs of activation of mania/hypomania and instruct them to report such symptoms to the healthcare provider
Psychosis and Other Neuropsychiatric Reactions
Inform patients that changes in mood including delusions, hallucinations, psychosis, concentration disturbances, paranoia, and confusion have occurred with use of bupropion, a component of AUVELITY. Instruct patients to notify a healthcare provider if they experience such symptoms
Angle-closure Glaucoma
Patients should be advised that taking AUVELITY can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle-closure glaucoma
Dizziness
Advise patients that AUVELITY may cause dizziness. Inform patients to take precautions to reduce the risk of falls, particularly for patients with motor impairment affecting gait or a history of falls. Caution patients about operating hazardous machinery, including motor vehicles, until they know how they will be affected by AUVELITY
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of AUVELITY with SSRIs or tricyclic antidepressants. Instruct patients to contact their health care provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome
Embryo-fetal Toxicity
Advise pregnant females and females of reproductive potential of the potential risk to a fetus
Lactation
Advise patients not to breastfeed during treatment with AUVELITY and for 5 days after the final dose
Administration Information
Advise patients not to take more than two tablets in the same day and to allow at least an 8-hour interval between doses
Drug Interactions
Inform patients that AUVELITY increases the risk of drug interactions. Instruct patients to inform their healthcare provider about all the medications that they are taking before taking AUVELITY. Before taking any new medications, patients should tell their healthcare provider that they are taking AUVELITY.
Advise patients that AUVELITY can increase adverse neuropsychiatric reactions or reduce alcohol tolerance and to avoid or limit use of alcohol during treatment with AUVELITY
Bupropion-Containing Products
Inform patients that AUVELITY contains bupropion which is an active ingredient in medications for other uses. Advise patients to inform their healthcare provider of all the medications that they are taking, including other bupropion-containing products
Dextromethorphan-Containing Products
Inform patients that AUVELITY contains dextromethorphan which is an active ingredient in medications for other uses. Advise patients to inform their healthcare provider of all the medications that they are taking, including other dextromethorphan-containing products
Distributed by:
Axsome Therapeutics, Inc.
AXSOME THERAPEUTICS and AUVELITY are trademarks or registered trademarks of Axsome Therapeutics, Inc. in the United States and other countries.
For patent information: www.axsome.com/IP
©2025 Axsome Therapeutics, Inc. All rights reserved.
AUV-USPI-003.000-20251119
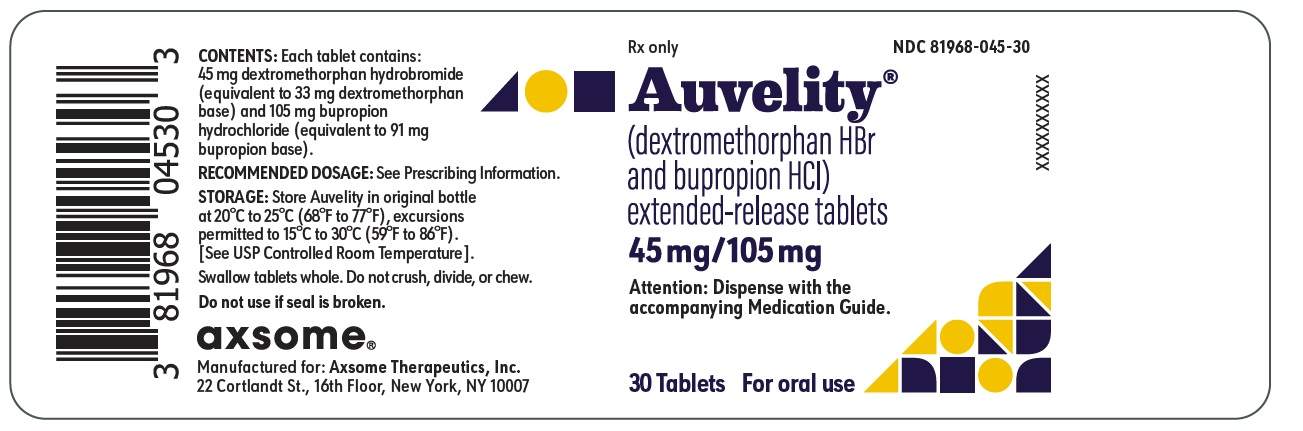
10PRINCIPAL DISPLAY PANEL - NDC: 81968-045-30 - 30-count Bottle Label
Rx only
NDC 81968-045-30
Auvelity
(dextromethorphan HBr and bupropion HCl)
extended-release tablets
45mg/105mg
Attention: Dispense with the accompanying Medication Guide.
30 Tablets
For oral use
11PRINCIPAL DISPLAY PANEL - NDC: 81968-045-60 - 60-count Bottle Label
Rx only
NDC 81968-045-60
Auvelity®
(dextromethorphan HBr and bupropion HCl)
extended-release tablets
45mg/105mg
Attention: Dispense with the accompanying Medication Guide.
60 Tablets
For oral use
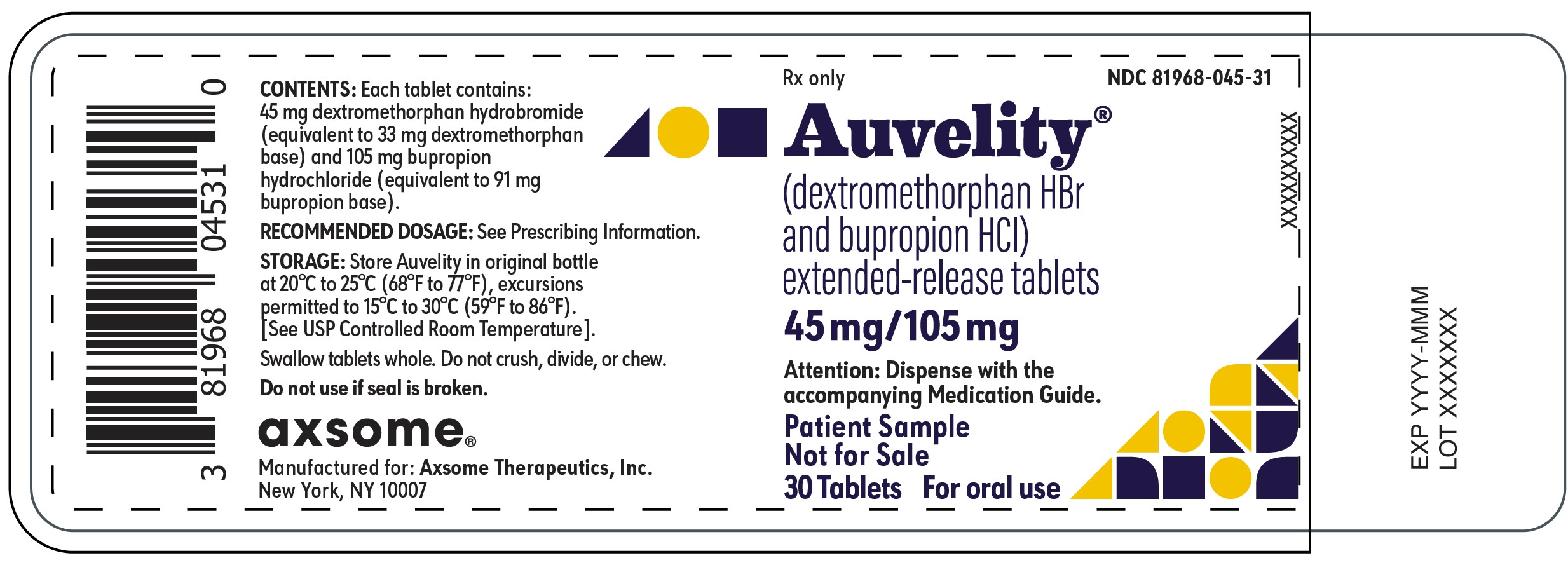
12PRINCIPAL DISPLAY PANEL - NDC: 81968-045-31 - 30-count Physician Sample Bottle Label
Rx only
NDC 81968-045-31
Auvelity®
(dextromethorphan HBr and bupropion HCl)
extended-release tablets
45mg/105mg
Attention: Dispense with the accompanying Medication Guide.
Patient Sample
Not for Sale
30 Tablets
For oral use
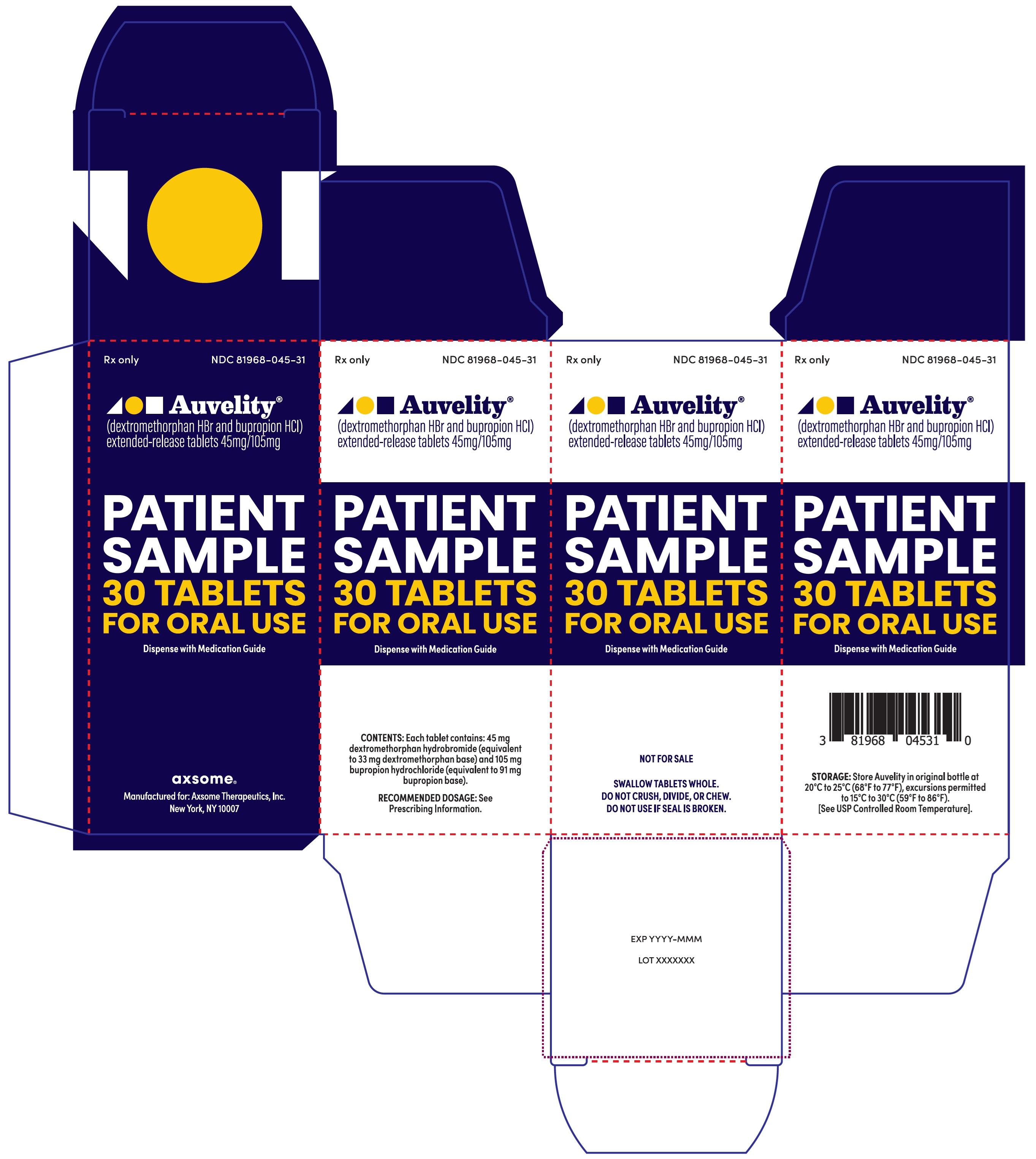
13PRINCIPAL DISPLAY PANEL - NDC: 81968-045-31 - 30-count Physician Sample Carton Label
Rx only
NDC 81968-045-31
Auvelity®
(dextromethorphan HBr and bupropion HCl)
extended-release tablets 45mg/105mg
Patient Sample
30 Tablets
For oral use
Dispense with Medication Guide
14PRINCIPAL DISPLAY PANEL - NDC: 81968-045-14 - 14-count Physician Sample Bottle Label
Rx only
NDC 81968-045-14
Auvelity®
(dextromethorphan HBr and bupropion HCl)
extended-release tablets
45mg/105mg
Attention: Dispense with the accompanying Medication Guide.
Patient Sample
Not for Sale
14 Tablets
For oral use
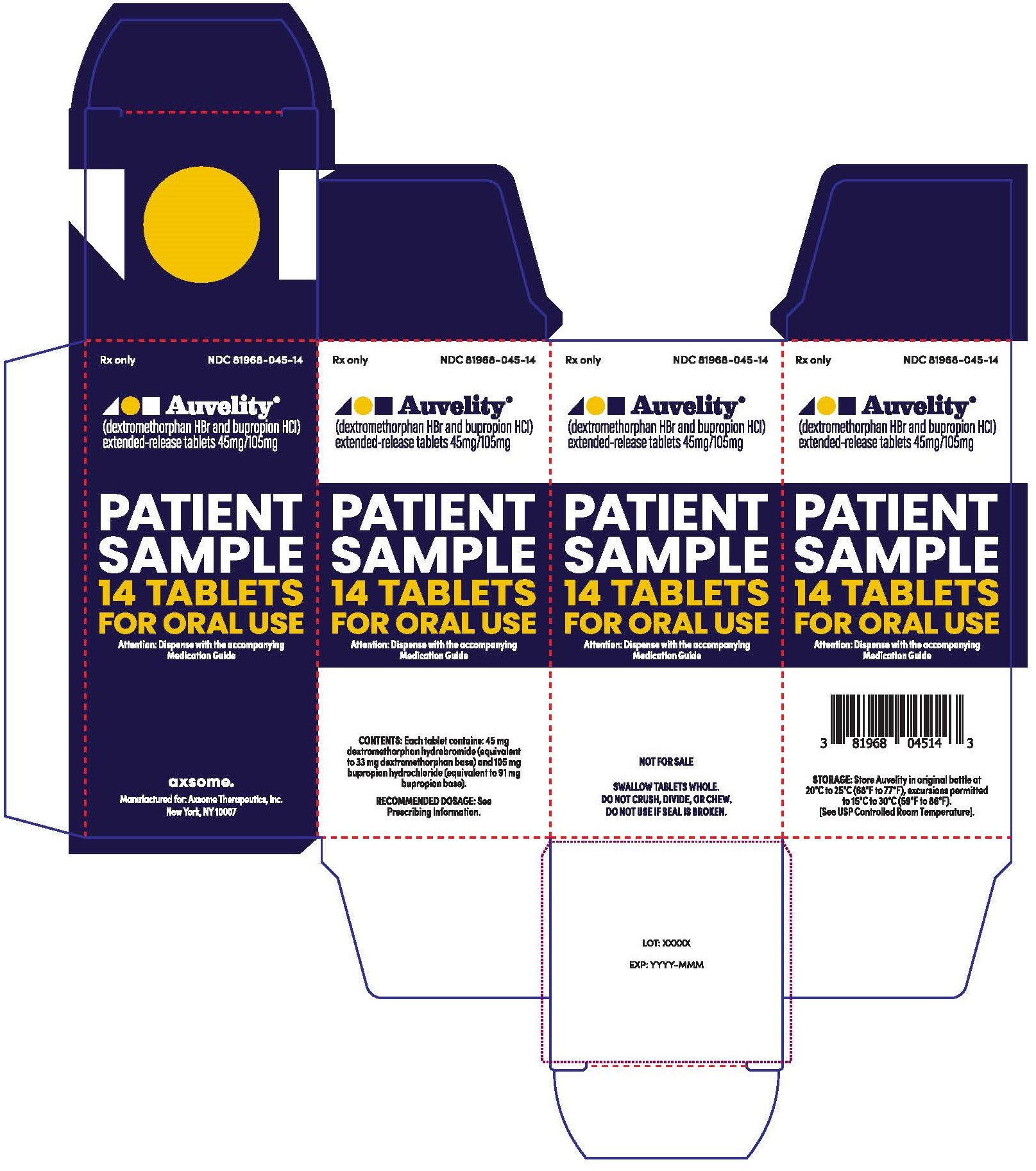
15PRINCIPAL DISPLAY PANEL - NDC: 81968-045-14 - 14-count Physician Sample Carton Label
Rx only
NDC 81968-045-31
Auvelity®
(dextromethorphan HBr and bupropion HCl)
extended-release tablets 45mg/105mg
Patient Sample
30 Tablets
For oral use
Dispense with Medication Guide