Simulect
What is Simulect (Basiliximab)?
For people receiving a life-saving organ transplant, one of the biggest challenges isn’t the surgery itself, it’s what comes afterward. The body’s immune system naturally tries to reject a new organ, seeing it as foreign. Without proper medication, this rejection can cause the transplanted kidney or liver to fail. Simulect (basiliximab) is a medication designed to prevent this, helping patients keep their new organ healthy and functioning for years to come.
Simulect is a monoclonal antibody used to prevent acute organ rejection in people receiving a kidney transplant. It is given as part of an immunosuppressive regimen, usually alongside other medications such as corticosteroids and calcineurin inhibitors (like cyclosporine or tacrolimus). Developed by Novartis and approved by the U.S. Food and Drug Administration (FDA), Simulect is a targeted therapy that works by calming the immune system in a precise way reducing the risk of rejection without overly weakening the body’s natural defenses.
What does Simulect do?
Simulect helps prevent the immune system from attacking a transplanted organ, most often a kidney. When someone receives an organ from a donor, their immune system identifies it as foreign and tries to destroy it. This immune attack, called acute rejection, can occur within days or weeks after transplantation and is a leading cause of early transplant failure.
Simulect is given around the time of the transplant surgery to lower this risk. It is not used to treat ongoing rejection but rather to prevent rejection from occurring in the first place. The drug works best when used in combination with other long-term immunosuppressants that maintain immune control over time.
Clinical studies have shown that patients who received Simulect as part of their transplant regimen had significantly lower rates of acute kidney rejection compared with those who did not. By improving early transplant outcomes, Simulect plays a vital role in helping patients recover safely and maintain good organ function (FDA, 2024; NIH, 2024).
How does Simulect work?
Simulect (basiliximab) is a monoclonal antibody, a laboratory-made protein that specifically targets one part of the immune system. It works by blocking the interleukin-2 receptor (IL-2R) found on the surface of certain white blood cells called T-lymphocytes.
T-lymphocytes (T-cells) are central players in the immune response. After a transplant, these cells recognize the new organ as foreign and become activated to attack it. Interleukin-2 (IL-2) is a key chemical signal that tells T-cells to multiply and mount this attack.
Simulect binds tightly to the IL-2R, preventing IL-2 from activating T-cells. By stopping this activation early in the process, Simulect helps prevent the chain reaction that leads to rejection. Importantly, it does this without completely shutting down the immune system, making it a selective and well-tolerated option for transplant patients.
Clinically, this mechanism matters because it reduces the need for high doses of other immunosuppressants, which often carry more severe long-term side effects like infections or kidney toxicity.
Simulect side effects
Most patients tolerate Simulect well, especially since it is given only for a short period around the time of transplantation. However, like all medications that affect the immune system, it can cause side effects.
Common side effects may include:
- Constipation or nausea
- Headache
- Tremors
- Swelling in the hands, feet, or ankles (fluid retention)
- High blood pressure
- Anemia or changes in blood cell counts
Serious but less common side effects:
- Severe allergic reaction (rash, itching, swelling, difficulty breathing)
- Signs of infection such as fever, chills, or sore throat
- Chest pain or irregular heartbeat
- Unusual bleeding or bruising
Simulect, combined with other immunosuppressants, poses a low risk of infections or rare cancers like lymphoma or skin cancer, especially with proper dosing and monitoring.
Who should avoid Simulect:
Basiliximab is contraindicated for those with severe allergic reactions to it or its components. It’s not recommended for non-kidney organ transplants unless directed by a specialist.
Patients should seek immediate medical attention for breathing difficulties, facial swelling, or anaphylaxis post-administration.
Simulect dosage
Simulect, an IV infusion given in a hospital, is not a daily medication. Most transplant patients receive two doses: one before surgery and one a few days later, to protect against organ rejection. Doctors monitor vital signs, kidney function, and infection markers for safety and effectiveness..
Simulect doesn’t need blood-level monitoring due to predictable absorption and clearance. However, regular checkups and lab tests are still necessary to assess immune function and organ health. Older adults and those with liver or kidney impairment typically don’t require dose adjustments but need close supervision to prevent infections or complications.
Does Simulect have a generic version?
As of 2025, Simulect (basiliximab) does not have an FDA-approved generic version in the United States. It is only sold as the brand-name medication manufactured by Novartis Pharmaceuticals. However, international versions may exist in other markets.
Biosimilar basiliximab versions are being developed globally, offering comparable safety, purity, and potency to the original drug. These biosimilars, once approved, will provide more affordable options for hospitals and transplant programs, maintaining clinical effectiveness and meeting strict quality standards.
Conclusion
Simulect (basiliximab) is a vital medication that helps protect transplanted kidneys from immune system attack. By blocking a key pathway that activates immune cells, it helps patients avoid early organ rejection and improves long-term transplant success.
Administered under medical supervision, Simulect is generally well-tolerated and vital for transplant medicine. Its targeted mechanism and strong safety record help patients focus on recovery rather than rejection.
Simulect is part of a comprehensive treatment plan. Patients must maintain regular follow-ups, take prescribed medications, and communicate with their healthcare team for optimal outcomes.
References
- U.S. Food and Drug Administration (FDA). (2024). Simulect (basiliximab) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Basiliximab injection: Uses and side effects. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Basiliximab: Drug information. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Immunosuppressive therapy in organ transplantation. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This phase II trials studies the effects of yttrium-90 labeled anti-CD25 monoclonal antibody combined with BEAM chemotherapy conditioning in treating patients with Hodgkin lymphoma that does not response to treatment (refractory) or has come back (relapsed). Yttrium-90-labeled anti-CD25 is an antibody (proteins made by the immune system to fight infections) that is attached to a radioactive substa...
Summary: This study aims to evaluate an integrated treatment protocol for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia (Ph- ALL), combining induction chemotherapy, consolidation therapy, and allogeneic hematopoietic stem cell transplantation (allo-HSCT) to improve treatment efficacy and survival rates. The single-arm, open-label, multicenter study will enroll 50 newly diagnosed...
Summary: This is a single-center, single-arm Phase Investigational Intervention Trial (IIT) clinical trial aimed at evaluating the safety and efficacy of allogeneic regenerative islet transplantation for the treatment of brittle type 1 diabetes mellitus. Eighteen patients with brittle type 1 diabetes mellitus, who have inadequate blood glucose control despite intensified exogenous insulin therapy, will be ...
Related Latest Advances
Brand Information
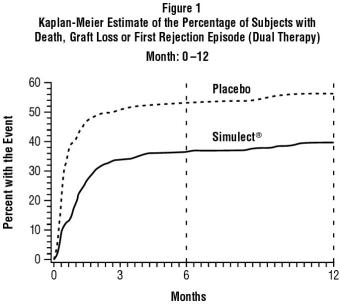
- Kahan, B.D., Rajagopalan P.R. and Hall M., Transplantation, 67, 276-284 (1999).
- Nashan, B., Moore R., Amlot P., Schmidt A.-G., Abeywickrama K. and Soulillou J.-P., Lancet 350, 1193-1198 (1997).