Brand Name
Zyvox
Generic Name
Linezolid
View Brand Information FDA approval date: April 18, 2000
Classification: Oxazolidinone Antibacterial
Form: Injection, Tablet, Granule, Powder, Suspension
What is Zyvox (Linezolid)?
Linezolid Injection is an oxazolidinone-class antibacterial indicated in adults and children for the treatment of the following infections caused by susceptible Gram-positive bacteria: Nosocomial pneumonia.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
Zyvox (linezolid)
1DOSAGE FORMS AND STRENGTHS
ZYVOX I.V. Injection: 600 mg/300 mL (2 mg/mL) linezolid single-dose, ready-to-use flexible plastic infusion bags in a foil laminate overwrap.
2ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression [
- Peripheral and Optic Neuropathy [
- Serotonin Syndrome [
- Clostridioides difficile-Associated Diarrhea [see ]
- Lactic Acidosis [
- Convulsions [
- Rhabdomyolysis [
- Hypoglycemia [
- Hyponatremia and/or Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) [
2.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
2.2Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ZYVOX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Anaphylaxis, angioedema, bullous skin disorders including severe cutaneous adverse reactions (SCAR) such as toxic epidermal necrolysis and Stevens-Johnson syndrome, and hypersensitivity vasculitis.
- Myelosuppression (including anemia, leukopenia, pancytopenia, and thrombocytopenia). Thrombocytopenia has been reported more often in patients with severe renal impairment and in patients with moderate to severe hepatic impairment [
- Peripheral neuropathy, and optic neuropathy sometimes progressing to loss of vision [
- Serotonin syndrome has been reported in patients receiving concomitant serotonergic agents, including antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and opioids, and ZYVOX [
- Lactic acidosis [
- Convulsions [
- Rhabdomyolysis [
- Hypoglycemia, including symptomatic episodes [
- Hyponatremia and/or Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) [
- Superficial tooth discoloration and tongue discoloration have been reported with the use of linezolid. The tooth discoloration was removable with professional dental cleaning (manual descaling) in cases with known outcome.
3OVERDOSAGE
In the event of overdosage, supportive care is advised, with maintenance of glomerular filtration. Hemodialysis may facilitate more rapid elimination of linezolid. In a Phase 1 clinical trial, approximately 30% of a dose of linezolid was removed during a 3-hour hemodialysis session beginning 3 hours after the dose of linezolid was administered. Data are not available for removal of linezolid with peritoneal dialysis or hemoperfusion. Clinical signs of acute toxicity in animals were decreased activity and ataxia in rats and vomiting and tremors in dogs treated with 3,000 mg/kg/day and 2,000 mg/kg/day, respectively.
4DESCRIPTION
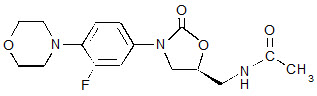
ZYVOX I.V. Injection contains linezolid, which is a synthetic antibacterial agent of the oxazolidinone class. The chemical name for linezolid is (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl]-acetamide.
The empirical formula is C
ZYVOX I.V. Injection is supplied as a ready-to-use sterile isotonic solution for intravenous infusion. Each mL contains 2 mg of linezolid. Inactive ingredients are dextrose monohydrate 50.24 mg/mL in an aqueous vehicle for intravenous administration, sodium citrate dihydrate 1.64 mg/mL, and citric acid anhydrous 0.85 mg/mL. Sodium hydroxide NF and/or hydrochloric acid NF are used to adjust the pH. The sodium (Na
5HOW SUPPLIED/STORAGE AND HANDLING
ZYVOX I.V. Injection is available in single-dose, ready-to-use flexible plastic infusion bags in a foil laminate overwrap. The infusion bags and ports are not made with natural rubber latex. The infusion bags are available in the following package sizes:
6PRINCIPAL DISPLAY PANEL - 300 mL Bag Label
NDC 0009-7807-01
Store at 25°C (77°F);
pH adjusted to 4.8 with sodium
PREMIERProRx
freeflex
1234567890
7
Distributed by:
PREMIERProRx
LOT

7PRINCIPAL DISPLAY PANEL - 300 mL Overwrap Label
NDC 0009-7807-01
Store at 25°C (77°F); excursions
For intravenous administration
7
Distributed by Pharmacia & Upjohn Co
FSU 0703 01-78-26-050F
LOT

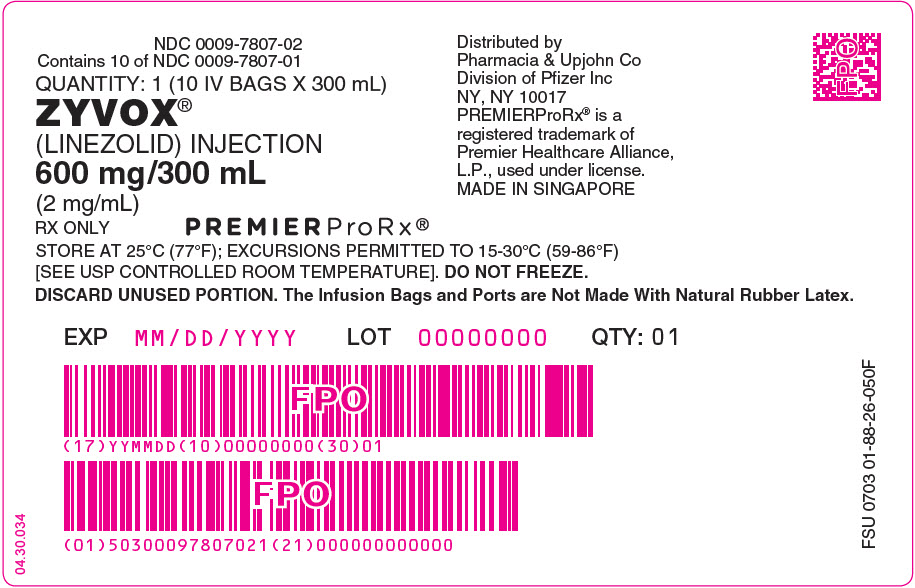
8PRINCIPAL DISPLAY PANEL - 300 mL Bag Box Label
NDC 0009-7807-02
RX ONLY
STORE AT 25°C (77°F); EXCURSIONS PERMITTED TO 15-30°C (59-86°F)
Distributed by
EXP YYYY-MM
FSU 0703 01-88-26-050H