Glycol-3350
What is GoLYTELY (Glycol-3350)?
Preparing for a colonoscopy or certain digestive procedures can be stressful especially when it involves cleansing the bowel beforehand. For many people, this step feels intimidating, but it’s essential for accurate medical results and a smooth experience. GoLYTELY (polyethylene glycol 3350 with electrolytes) is a bowel preparation solution that helps patients safely and effectively clean out their colon before procedures such as a colonoscopy.
GoLYTELY belongs to a class of medications known as osmotic laxatives. It works by helping the intestines retain water, softening and flushing stool out of the body. This ensures the colon is completely clear, allowing doctors to see the intestinal walls clearly during an exam. Approved by the U.S. Food and Drug Administration (FDA), GoLYTELY has been used for decades as a trusted, medically supervised bowel preparation. While not a pleasant process, it’s a necessary and important part of preventive care helping detect and prevent serious conditions such as colorectal cancer.
What does GoLYTELY do?
GoLYTELY is prescribed to clean the bowel before diagnostic procedures, most commonly a colonoscopy. During a colonoscopy, a doctor uses a thin, flexible tube with a camera to examine the lining of the colon and rectum. For accurate results, the colon must be completely empty of stool.
By causing frequent bowel movements, GoLYTELY flushes out any waste or residue from the digestive tract. Patients can expect several loose, watery stools over a few hours after starting the preparation. The process may feel inconvenient, but it is temporary and it’s vital for ensuring that the colonoscopy can be performed safely and accurately.
Beyond colonoscopy prep, GoLYTELY is sometimes used under medical supervision to treat severe constipation or to clear the intestines before certain surgeries or imaging studies. Studies and decades of clinical use have shown it to be highly effective, with most patients achieving adequate bowel cleansing when used as directed (Mayo Clinic, 2024).
How does GoLYTELY work?
GoLYTELY works through a simple yet powerful mechanism. It contains polyethylene glycol 3350 (PEG-3350), an osmotic agent combined with electrolytes such as sodium, potassium, and bicarbonate.
When taken as directed, PEG-3350 draws water into the colon without being absorbed by the body. This extra water softens the stool and triggers bowel movements that gradually empty the intestines. The added electrolytes replace essential salts and prevent dehydration or mineral imbalance during the cleansing process.
Clinically, this mechanism is significant because it provides a complete colon cleanse without major fluid or electrolyte loss. This makes GoLYTELY safer and more balanced than older laxatives or home remedies that could cause dehydration or dangerous sodium shifts.
In short, GoLYTELY helps the body flush itself naturally keeping hydration stable while preparing the digestive system for medical evaluation.
GoLYTELY side effects
Like all bowel preparation medications, GoLYTELY can cause temporary side effects, most of which occur as part of its cleansing action.
Common side effects may include:
- Nausea or mild stomach cramping
- Bloating or gas
- Chills or mild dizziness
- A feeling of fullness during drinking
- Frequent, watery stools
These effects are expected and generally subside once the bowel is completely emptied. To minimize discomfort, patients are advised to drink the solution slowly and stay near a restroom.
Less common but serious side effects may include:
- Severe dehydration (signs include dizziness, confusion, or decreased urination)
- Persistent vomiting
- Chest pain, irregular heartbeat, or muscle weakness (possible electrolyte imbalance)
- Signs of an allergic reaction such as rash, itching, or swelling
GoLYTELY requires close medical supervision for patients with kidney disease, heart conditions, or electrolyte disorders. Avoid use if you have a history of bowel obstruction, GI perforation, or inflammatory bowel disease flare-ups, unless approved by a doctor.
If any severe or persistent side effects occur, especially fainting, irregular heartbeat, or signs of dehydration, patients should seek immediate medical attention.
GoLYTELY dosage
GoLYTELY is a powder mixed with water and consumed orally over several hours, following a strict schedule. Adhering precisely to timing, speed, and liquid amount is crucial for bowel cleansing. Most preparations also involve dietary restrictions, like a clear-liquid diet the day before and avoiding solid foods.
Stay hydrated with clear fluids before and after GoLYTELY. Monitor for dehydration (lightheadedness, dry mouth). Older adults and those with kidney/heart disease may need extra electrolyte monitoring. Do not combine with other laxatives unless advised by a healthcare professional.
Does GoLYTELY have a generic version?
Yes. Generic versions of GoLYTELY containing polyethylene glycol 3350 and electrolytes are widely available and FDA-approved. These generic products are equally effective and safe as the brand-name version when prepared and used according to instructions.
Generic versions of bowel prep include “PEG-3350 with electrolytes” or “polyethylene glycol electrolyte solution.” Brand names for similar preparations are NuLYTELY, CoLyte, and TriLyte. Patients must confirm their prescribed version with their healthcare provider and follow specific instructions. Adherence to preparation directions is crucial for effective colon cleansing, regardless of brand or generic type.
Conclusion
GoLYTELY is a proven, effective, and medically supervised bowel cleansing solution designed to prepare the intestines for colonoscopy and other diagnostic procedures. By gently drawing water into the bowel and flushing out waste, it ensures a clear view of the colon for accurate results and safer care.
Though inconvenient to prepare, GoLYTELY uses a balanced formula to minimize dehydration and discomfort. Side effects are typically mild and temporary, with serious reactions being rare when used as prescribed. It is a safe, reliable, and essential tool for digestive health, enabling early detection of colon polyps, cancer, and other gastrointestinal conditions under professional guidance.
References
- Mayo Clinic. (2024). Polyethylene glycol and electrolytes (oral route) drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Polyethylene glycol-electrolyte solution: Uses and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- U.S. Food and Drug Administration (FDA). (2023). GoLYTELY prescribing information. Retrieved from https://www.accessdata.fda.gov
- National Institutes of Health (NIH). (2024). Bowel preparation for colonoscopy: Overview and safety considerations. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- Gastrointestinal (GI) obstruction
- Bowel perforation
- Toxic colitis or toxic megacolon
- Gastric retention
- Ileus
- Hypersensitivity to any component of GoLYTELY
- Renal impairment
- Colonic mucosal ulcerations and ischemic colitis
- Patients with significant gastrointestinal disease
- Aspiration
- Cardiovascular: arrhythmia, atrial fibrillation, peripheral edema, asystole, and acute pulmonary edema after aspiration
- Nervous system: tremor, seizure
- Hypersensitivity: Urticaria/rash, pruritus, dermatitis, rhinorrhea, dyspnea, chest and throat tightness, fever, angioedema, anaphylaxis and anaphylactic shock
- Gastrointestinal: Nausea, abdominal fullness and bloating are the most common adverse reactions (occurred in up to 50% of patients). Other less common adverse reactions include: abdominal cramps, vomiting, “butterfly-like” infiltrates on chest X-ray after vomiting and aspirating PEG, anal irritation, and upper GI bleeding from Mallory-Weiss Tear, esophageal perforation [usually with gastroesophageal reflux disease (GERD)].
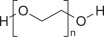
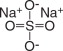
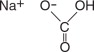
- When reconstituted with water to a volume of 4 liters, the solution contains 59 g/L PEG-3350, 5.69 g/L sodium sulfate, 1.69 g/L sodium bicarbonate, 1.47 g/L sodium chloride and 0.743 g/L potassium chloride.
- To reconstitute GoLYTELY with water prior to ingestion.
- Not to take other laxatives while they are taking GoLYTELY.
- Not to take oral medications within 1 hour before the start or during the administration of GoLYTELY.
- To take only clear liquids but avoid red and purple liquids.
- To consume water or other clear liquids during the bowel preparation and after completion of the bowel preparation up until 2 hours before the time of the colonoscopy.
- To follow the directions in the
- If they experience severe bloating, distention or abdominal pain, to slow or temporarily discontinue drinking the solution and to contact their healthcare provider.
- To contact their healthcare provider if they develop signs and symptoms of dehydration or if they experience altered consciousness or seizures.
- To discontinue administration of the solution and contact their healthcare provider if they develop symptoms of a hypersensitivity reaction