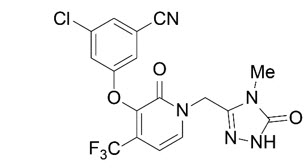
Pifeltro
What is Pifeltro (Doravirine)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: Phase III trial evaluating doravirine as an alternative to dolutegravir in treatment naïve people living with HIV-1 infection.
Summary: The goal of this study is to learn what happens to doravirine (DOR) and islatravir (ISL) in a healthy lactating female's body over time. Researchers want to learn if DOR and ISL are in breast milk.
Summary: The goal of this pilot, phase 2, single-arm, clinical trial is to assess the antiretroviral combination Doravirine (DOR)/Lamivudine (3TC)/Tenofovir Disproxyl Fumarate (TDF) in participants with suppressed HIV who previously developed M184V/I mutation that confers resistance to 3TC. The main question it aims to answer is to explore the rate of HIV suppression 24 weeks after the switch to DOR/3TC/TD...
Related Latest Advances
Brand Information
- with no prior antiretroviral treatment history;
- to replace the current antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen with no history of treatment failure and no known substitutions associated with resistance to doravirine
- the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, phenytoin
- the androgen receptor inhibitor enzalutamide
- the antimycobacterials rifampin, rifapentine
- the cytotoxic agent mitotane
- St. John's wort (
- Immune Reconstitution Syndrome