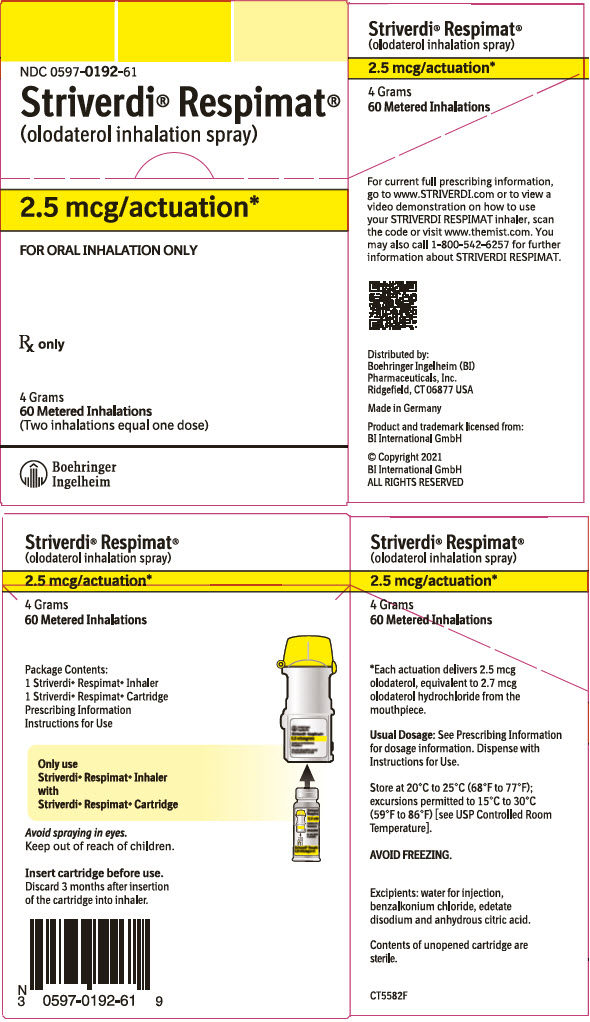
Striverdi Respimat
What is Striverdi Respimat (OLODATEROL Respimat)?
Living with chronic obstructive pulmonary disease (COPD) can make even simple tasks walking, climbing stairs, or carrying groceries feel exhausting. Breathing becomes a daily challenge, leaving many people struggling for air and energy. Striverdi Respimat (olodaterol Respimat) is designed to help people with COPD breathe easier, stay active, and regain confidence in their daily lives.
Striverdi Respimat is an inhalation medication that belongs to a class of drugs known as long-acting beta₂-agonists (LABAs). These medications work by relaxing the muscles around the airways, making it easier to move air in and out of the lungs. It is used as a maintenance treatment, meaning it helps manage COPD symptoms over time, rather than providing quick relief during sudden flare-ups. Approved by the U.S. Food and Drug Administration (FDA), Striverdi Respimat offers patients long-lasting, once-daily symptom control that improves lung function and quality of life.
What does Striverdi Respimat do?
Striverdi Respimat is prescribed to treat chronic obstructive pulmonary disease (COPD), which includes conditions such as chronic bronchitis and emphysema. COPD causes inflammation and narrowing of the airways, leading to coughing, wheezing, shortness of breath, and fatigue.
The medication helps by keeping airways open for a full 24 hours, making breathing easier and reducing daily symptoms. Patients often notice improved airflow, less shortness of breath, and increased ability to perform daily activities after regular use.
Striverdi Respimat is not a rescue inhaler and should not be used to treat sudden breathing problems. Instead, it works best when used consistently every day. Clinical studies have shown that olodaterol improves lung function and reduces breathlessness compared to placebo treatments, helping patients better manage their COPD and maintain long-term respiratory stability (NIH, 2024).
How does Striverdi Respimat work?
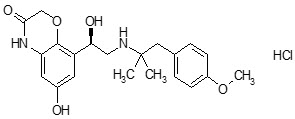
Striverdi Respimat contains olodaterol, a long-acting beta₂-adrenergic agonist (LABA). In simple terms, it helps the airways in the lungs stay relaxed and open.
Inside the lungs, the walls of the airways are surrounded by smooth muscles that can tighten due to inflammation or irritation. When this happens, airflow becomes restricted, making it difficult to breathe. Olodaterol attaches to beta₂ receptors on these airway muscles, stimulating them to relax. This relaxation widens the airways, a process known as bronchodilation allowing air to flow more freely.
This mechanism provides long-lasting relief, helping patients breathe easier throughout the day and night. The Respimat device delivers the medication as a fine mist inhaler, ensuring deep delivery into the lungs for maximum effectiveness. Clinically, this matters because maintaining open airways helps prevent exacerbations (flare-ups), reduce hospital visits, and improve overall lung function over time.
Striverdi Respimat side effects
Like all medications, Striverdi Respimat can cause side effects. Most are mild and temporary, but it’s important to be aware of them.
Common side effects may include:
- Cough or mild throat irritation
- Dizziness or headache
- Nasal congestion or sore throat
- Muscle cramps
- Joint or back pain
Serious side effects (less common):
- Chest pain or rapid heartbeat
- Severe dizziness or fainting
- Worsening breathing problems right after use
- Signs of allergic reaction such as rash, swelling, or difficulty breathing
Because Striverdi Respimat is a LABA, it should not be used as monotherapy for asthma, as this could increase the risk of asthma-related complications. However, it is safe and well-studied for COPD when used as directed.
People with heart conditions, high blood pressure, thyroid disorders, diabetes, or seizures should inform their doctor before starting the medication. Patients should seek immediate medical attention if they experience severe chest pain, irregular heartbeat, or sudden worsening of breathing.
Overall, most patients tolerate Striverdi Respimat well. Regular follow-up visits help ensure any side effects are identified and managed early.
Striverdi Respimat dosage
Striverdi Respimat is inhaled once daily at the same time for 24-hour airway opening. The slow mist aids deep inhalation, especially for those with reduced lung capacity. Follow healthcare provider instructions for proper use; do not exceed the dose or combine with other long-acting beta₂-agonists due to side effect risks.
Doctors may recommend periodic lung function tests and blood pressure monitoring to ensure the medication remains effective and well-tolerated. For older adults or those with underlying heart conditions, dosage adjustments are usually not necessary, but careful monitoring is advised.
Does Striverdi Respimat have a generic version?
As of 2025, Striverdi Respimat (olodaterol Respimat) does not currently have a generic version approved in the United States. It is available only as the brand-name product manufactured by Boehringer Ingelheim Pharmaceuticals.
Olodaterol is also in Stiolto Respimat with tiotropium for broader symptom control. A generic Striverdi Respimat will have the same active ingredient, strength, safety, and effectiveness. Patients can discuss cost with their healthcare provider.
Conclusion
Striverdi Respimat is a trusted, once-daily inhalation therapy for adults living with COPD. By relaxing airway muscles and improving airflow, it helps people breathe more easily and enjoy greater independence in daily life.
Striverdi Respimat is vital for long-term COPD management, reducing symptoms, preventing flare-ups, and improving lung function. Most patients experience better breathing and quality of life with proper use and medical supervision. Openly communicate with your doctor about its role in your care, side effects, and follow-up. This medication can significantly boost your confidence in living and breathing.
References
- U.S. Food and Drug Administration (FDA). (2024). Striverdi Respimat (olodaterol) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Olodaterol (inhalation route) drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Olodaterol inhalation: Uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Long-acting bronchodilators in COPD management. Retrieved from https://www.nih.gov
Approved To Treat
Related Clinical Trials
There is no clinical trials being done for this treatment
Brand Information
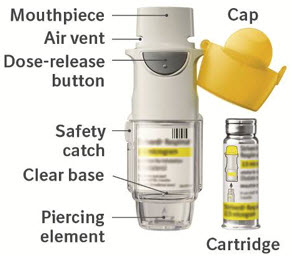
- Store STRIVERDI RESPIMAT at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze your STRIVERDI RESPIMAT cartridge and inhaler.
- If STRIVERDI RESPIMAT has not been used for more than 3 days, release 1 puff towards the ground.
- If STRIVERDI RESPIMAT has not been used for more than 21 days, repeat steps 4 to 6 under the "Prepare for first use" until a mist is visible. Then repeat steps 4 to 6 three more times.
- Keep your STRIVERDI RESPIMAT cartridge and inhaler out of the reach of children.
- Your inhaler contains 60 puffs (30 doses) if used as indicated (2 puffs once daily). If you have a sample, your inhaler contains 28 puffs (14 doses) if used as indicated (2 puffs once daily).
- The dose indicator shows approximately how much medicine is left.
- When the dose indicator enters the red area of the scale you need to get a refill; there is approximately medicine for 7 days left (if you have a sample, there is approximately medicine for 3 days left).
- When the dose indicator reaches the end of the red scale, your STRIVERDI RESPIMAT is empty and automatically locks. At this point, the clear base cannot be turned any further.
- Three months after insertion of cartridge, throw away the STRIVERDI RESPIMAT even if it has not been used, or when the inhaler is locked, or when it expires, whichever comes first.