Brand Name
Eraxis
Generic Name
Anidulafungin
View Brand Information FDA approval date: February 17, 2006
Classification: Echinocandin Antifungal
Form: Injection
What is Eraxis (Anidulafungin)?
ERAXIS is an echinocandin antifungal indicated for the treatment of the following infections: Candidemia and other forms of Candida infections in adults and pediatric patients .
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
Translational PKPD Modeling of Anti-infective Drugs in Children Treated in Pediatric Units on the Example of Selected Antibiotics and Antifungals.
Summary: Pharmacokinetic and pharmacodynamic modeling (PKPD) is becoming an essential tool for optimizing pharmacotherapy. Building mechanistic models allows determining the relationship between the dose, concentration, pharmacological effect, and side effects in various populations. The growing resistance to drugs among bacteria is a challenge for medicine, and the progress in pharmacometrics enables us t...
Impact of Capillary Leak and Hypoalbuminemia on PK/PD of Anidulafungin and Caspofungin in Critically Ill Patients
Summary: This prospective study will explore the pharmacokinetic exposure and pharmacodynamics of the echinocandins (caspofungin or anidulafungin) in critically ill patients.
Related Latest Advances
Brand Information
ERAXIS (anidulafungin)
1DOSAGE FORMS AND STRENGTHS
For injection: 50 mg, and 100 mg of anidulafungin as a white to off-white sterile lyophilized powder in a single-dose vial for reconstitution.
2CONTRAINDICATIONS
ERAXIS is contraindicated in:
- Patients with known hypersensitivity to anidulafungin, any component of ERAXIS, or other echinocandins
- Patients with known or suspected Hereditary Fructose Intolerance (HFI)
3ADVERSE REACTIONS
The following most
- Hepatic Adverse Reactions
- Anaphylactic and Hypersensitivity Reactions
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
3.2Post-marketing Experience
The following adverse reactions have been identified during post approval use of anidulafungin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune: Anaphylactic shock, anaphylactic reaction, bronchospasm [see .
4OVERDOSAGE
During clinical trials a single 400 mg dose of ERAXIS was inadvertently administered as a loading dose. No clinical adverse events were reported. In a study of 10 healthy subjects administered a loading dose of 260 mg followed by 130 mg daily; 3 of the 10 subjects experienced transient, asymptomatic transaminase elevations (≤3 × ULN)
Anidulafungin is not dialyzable.
The maximum non-lethal dose of anidulafungin in rats was 50 mg/kg, a dose which is equivalent to 10 times the recommended daily dose for esophageal candidiasis (50 mg/day) or equivalent to 5 times the recommended daily dose for candidemia and other
5DESCRIPTION
ERAXIS for Injection is a sterile, lyophilized product for intravenous (IV) infusion that contains anidulafungin. ERAXIS (anidulafungin) is a semi-synthetic lipopeptide synthesized from a fermentation product of
ERAXIS (anidulafungin) is 1-[(4R,5R)-4,5-dihydroxy-N
50 mg/vial – fructose (50 mg), mannitol (250 mg), polysorbate 80 (125 mg), tartaric acid (5.6 mg), and sodium hydroxide and/or hydrochloric acid for pH adjustment.
100 mg/vial – fructose (100 mg), mannitol (500 mg), polysorbate 80 (250 mg), tartaric acid (11.2 mg), and sodium hydroxide and/or hydrochloric acid for pH adjustment.
The empirical formula of anidulafungin is C
The structural formula is:

Prior to administration, ERAXIS for Injection requires reconstitution with sterile Water for Injection and subsequent dilution with either 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP (normal saline).
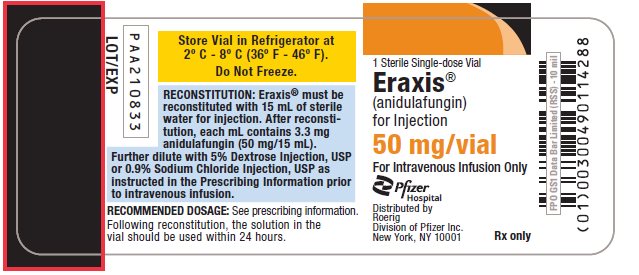
6PRINCIPAL DISPLAY PANEL - 50 mg Vial Label
1 Sterile Single-dose Vial
Eraxis®
(anidulafungin)
for Injection
(anidulafungin)
for Injection
50 mg/vial
For Intravenous Infusion Only
Pfizer
Distributed by
Rx only
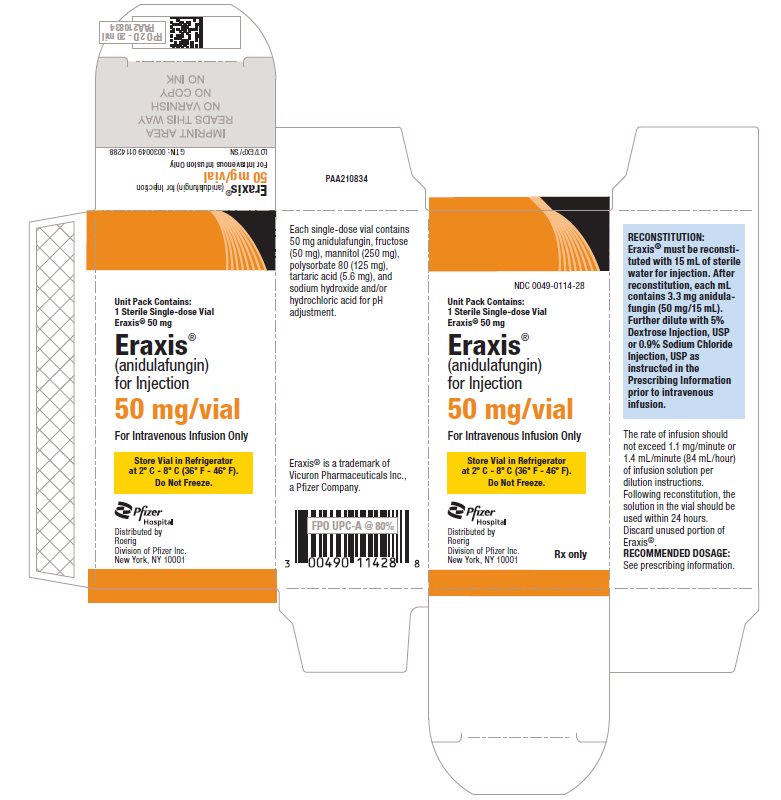
7PRINCIPAL DISPLAY PANEL - 50 mg Vial Carton
NDC 0049-0114-28
Unit Pack Contains:
1 Sterile Single-dose Vial
Eraxis® 50 mg
1 Sterile Single-dose Vial
Eraxis® 50 mg
Eraxis®
(anidulafungin)
for Injection
(anidulafungin)
for Injection
50 mg/vial
For Intravenous Infusion Only
Store Vial in Refrigerator
at 2° C - 8° C (36° F - 46° F).
Do Not Freeze.
at 2° C - 8° C (36° F - 46° F).
Do Not Freeze.
Pfizer
Distributed by
Rx only
8PRINCIPAL DISPLAY PANEL - 100 mg Vial Label
1 Sterile Single-dose Vial
Eraxis®
(anidulafungin)
for Injection
(anidulafungin)
for Injection
100 mg/vial
For Intravenous Infusion Only
Pfizer
Distributed by
Rx only

9PRINCIPAL DISPLAY PANEL - 100 mg Vial Carton
NDC 0049-0116-28
Unit Pack Contains:
1 Sterile Single-dose Vial
Eraxis® 100 mg
1 Sterile Single-dose Vial
Eraxis® 100 mg
Eraxis®
(anidulafungin)
for Injection
(anidulafungin)
for Injection
100 mg/vial
For Intravenous Infusion Only
Store Vial in Refrigerator
at 2° C - 8° C (36° F - 46° F).
Do Not Freeze.
at 2° C - 8° C (36° F - 46° F).
Do Not Freeze.
Pfizer
Distributed by
Rx only





