Brand Name
Fosamprenavir
View Brand InformationFDA approval date: September 18, 2017
Classification: Protease Inhibitor
Form: Tablet
What is Fosamprenavir?
Fosamprenavir calcium tablets are indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus infection. The following points should be considered when initiating therapy with fosamprenavir plus ritonavir in protease inhibitor-experienced patients: The protease inhibitor-experienced patient trial was not large enough to reach a definitive conclusion that fosamprenavir plus ritonavir and lopinavir plus ritonavir are clinically equivalent [see Clinical Studies (1.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
Fosamprenavir Calcium (fosamprenavir calcium)
1INDICATIONS AND USAGE
Fosamprenavir calcium tablets are indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection.
The following points should be considered when initiating therapy with fosamprenavir plus ritonavir in protease inhibitor-experienced patients:
- The protease inhibitor-experienced patient trial was not large enough to reach a definitive conclusion that fosamprenavir plus ritonavir and lopinavir plus ritonavir are clinically equivalent
- Once-daily administration of fosamprenavir plus ritonavir is not recommended for adult protease inhibitor-experienced patients or any pediatric patients
- Dosing of fosamprenavir plus ritonavir is not recommended for protease inhibitor-experienced pediatric patients younger than 6 months
2DOSAGE FORMS AND STRENGTHS
Fosamprenavir Calcium Tablets, USP are available containing 700 mg of fosamprenavir as fosamprenavir calcium, USP.
- The 700 mg tablets are pink, film-coated, modified capsule shaped, unscored tablets debossed with
3CONTRAINDICATIONS
- Fosamprenavir calcium tablets are contraindicated in patients with previously demonstrated clinically significant hypersensitivity (e.g., Stevens-Johnson syndrome) to any of the components of this product or to amprenavir.
- Fosamprenavir calcium tablets are contraindicated when coadministered with drugs that are highly dependent on cytochrome P450 (CYP)3A4 for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events. These drugs and other contraindicated drugs (which may lead to reduced efficacy of fosamprenavir and possible resistance) are listed below
- Fosamprenivir is contraindicated when coadministered with the following drugs:
4ADVERSE REACTIONS
- Severe or life-threatening skin reactions have been reported with the use of fosamprenavir
- The most common moderate to severe adverse reactions in clinical trials of fosamprenavir were diarrhea, rash, nausea, vomiting, and headache.
- Treatment discontinuation due to adverse events occurred in 6.4% of subjects receiving fosamprenavir and in 5.9% of subjects receiving comparator treatments. The most common adverse reactions leading to discontinuation of fosamprenavir (incidence less than or equal to 1% of subjects) included diarrhea, nausea, vomiting, AST increased, ALT increased, and rash.
4.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
4.1.1Adult Trials
The data for the 3 active-controlled clinical trials described below reflect exposure of 700 HIV-1– infected subjects to fosamprenavir calcium tablets, including 599 subjects exposed to fosamprenavir for greater than 24 weeks, and 409 subjects exposed for greater than 48 weeks. The population age ranged from 17 to 72 years. Of these subjects, 26% were female, 51% white, 31% black, 16% American Hispanic, and 70% were antiretroviral-naive. Sixty-one percent received fosamprenavir 1,400 mg once daily plus ritonavir 200 mg once daily; 24% received fosamprenavir 1,400 mg twice daily; and 15% received fosamprenavir 700 mg twice daily plus ritonavir 100 mg twice daily.
Selected adverse reactions reported during the clinical efficacy trials of fosamprenavir are shown in Tables 2 and 3. Each table presents adverse reactions of moderate or severe intensity in subjects treated with combination therapy for up to 48 weeks.
Skin rash (without regard to causality) occurred in approximately 19% of subjects treated with fosamprenavir in the pivotal efficacy trials. Rashes were usually maculopapular and of mild or moderate intensity, some with pruritus. Rash had a median onset of 11 days after initiation of fosamprenavir and had a median duration of 13 days. Skin rash led to discontinuation of fosamprenavir in less than 1% of subjects. In some subjects with mild or moderate rash, dosing with fosamprenavir was often continued without interruption; if interrupted, reintroduction of fosamprenavir generally did not result in rash recurrence.
The percentages of subjects with Grade 3 or 4 laboratory abnormalities in the clinical efficacy trials of fosamprenavir are presented in Tables 4 and 5.
The incidence of Grade 3 or 4 hyperglycemia in antiretroviral-naive subjects who received fosamprenavir in the pivotal trials was less than 1%.
4.1.2Pediatric Trials
Fosamprenavir with and without ritonavir was studied in 237 HIV-1– infected pediatric subjects aged at least 4 weeks to 18 years in 3 open-label trials; APV20002, APV20003, and APV29005
The frequency of vomiting among pediatric subjects receiving fosamprenavir twice daily with ritonavir was 20% in subjects aged at least 4 weeks to younger than 2 years and 36% in subjects aged 2 to 18 years compared with 10% in adults. The frequency of vomiting among pediatric subjects receiving fosamprenavir twice daily without ritonavir was 60% in subjects aged 2 to 5 years compared with 16% in adults.
The median duration of drug-related vomiting episodes in APV29005 was 1 day (range: 1 to 3 days), in APV20003 was 16 days (range: 1 to 38 days), and in APV20002 was 9 days (range: 4 to 13 days). Vomiting was treatment limiting in 4 pediatric subjects across all 3 trials.
The incidence of Grade 3 or 4 neutropenia (neutrophils less than 750 cells per mm
4.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of fosamprenavir. Because these reactions are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to fosamprenavir.
Cardiac Disorders: Myocardial infarction.
Metabolism and Nutrition Disorders: Hypercholesterolemia.
Nervous System Disorders: Oral paresthesia.
Skin and Subcutaneous Tissue Disorders: Angioedema.
Urogenital: Nephrolithiasis.
5OVERDOSAGE
In a healthy volunteer repeat-dose pharmacokinetic trial evaluating high-dose combinations of fosamprenavir plus ritonavir, an increased frequency of Grade 2/3 ALT elevations (greater than 2.5 x ULN) was observed with fosamprenavir 1,400 mg twice daily plus ritonavir 200 mg twice daily (4 of 25 subjects). Concurrent Grade 1/2 elevations in AST (greater than 1.25 x ULN) were noted in 3 of these 4 subjects. These transaminase elevations resolved following discontinuation of dosing.
There is no known antidote for fosamprenavir. It is not known whether amprenavir can be removed by peritoneal dialysis or hemodialysis, although it is unlikely as amprenavir is highly protein bound. If overdosage occurs, the patient should be monitored for evidence of toxicity and standard supportive treatment applied as necessary.
6DESCRIPTION
Fosamprenavir calcium, USP is a prodrug of amprenavir, an inhibitor of HIV protease. The chemical name of fosamprenavir calcium is (3
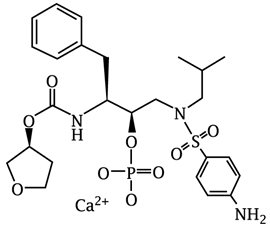
Fosamprenavir calcium is a white to cream color powder with a solubility of approximately 0.31 mg per mL in water at 25°C.
Fosamprenavir calcium tablets, USP are available for oral administration in a strength of 700 mg of fosamprenavir as fosamprenavir calcium (equivalent to approximately 600 mg of amprenavir). Each 700-mg tablet contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, red iron oxide, silicified microcrystalline cellulose, titanium dioxide and triacetin.
Meets USP Dissolution Test 2
7HOW SUPPLIED/STORAGE AND HANDLING
Fosamprenavir Calcium Tablets, USP are available containing 700 mg of fosamprenavir as fosamprenavir calcium, USP.
The 700 mg tablets are pink, film-coated, modified capsule shaped, unscored tablets debossed with
NDC 0378-3520-91
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Keep container tightly closed.
Dispense in original container.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Drug Interactions: A statement to patients and healthcare providers is included on the product’s bottle label: ALERT: Find out about medicines that should NOT be taken with Fosamprenavir Calcium Tablets.
Fosamprenavir calcium tablets may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, particularly St. John’s wort.
Advise patients receiving PDE5 inhibitors that they may be at an increased risk of PDE5 inhibitor-associated adverse events, including hypotension, visual changes, and priapism, and should promptly report any symptoms to their healthcare provider
Contraception:Instruct patients receiving combined hormonal contraception to use an effective alternative contraceptive method or an additional barrier method during therapy with fosamprenavir calcium tablets because hormonal levels may decrease, and if used in combination with fosamprenavir calcium tablets and ritonavir, liver enzyme elevations may occur [see .
Severe Skin Reactions: Advise patients that skin reactions ranging from mild to severe, including Stevens-Johnson Syndrome, have been reported with fosamprenavir calcium tablets. Advise patients to discontinue fosamprenavir calcium tablets immediately for severe or life-threatening skin reactions or for moderate rashes accompanied by systemic symptoms [see .
Sulfa Allergy: Advise patients to inform their healthcare provider if they have a sulfa allergy. The potential for cross-sensitivity between drugs in the sulfonamide class and fosamprenavir is unknown [see .
Hepatic Toxicity: Advise patients that it is recommended to have laboratory testing before and during therapy as patients with underlying hepatitis B or C or marked elevations of transaminases prior to treatment may be at increased risk for developing or worsening transaminase elevations with use of fosamprenavir calcium tablets, particularly at higher than recommended doses which should not be used [see .
Immune Reconstitution Syndrome:Advise patients to inform their healthcare provider immediately of any signs or symptoms of infection as inflammation from previous infection may occur soon after combination antiretroviral therapy, including when fosamprenavir calcium tablets are started [see .
Increase in Body Fat:Inform patients that an increase of body fat may occur in patients receiving protease inhibitors, including fosamprenavir calcium tablets, and that the cause and long-term health effects of these conditions are not known at this time [see .
Lipid Elevations:Advise patients that it is recommended to have laboratory testing before and during therapy as increases in the concentration of triglycerides and cholesterol have been reported with use of fosamprenavir calcium tablets [see .
Pregnancy Registry:Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to fosamprenavir calcium tablets during pregnancy [see .
Lactation: Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in the breast milk [see .
Missed Dose: Instruct patients that if they miss a dose of fosamprenavir calcium tablets, to take it as soon as they remember. Advise patients not to double their next dose or take more than the prescribed dose [see .
9Patient Information
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Manufactured by:
75099448
Revised: 9/2023
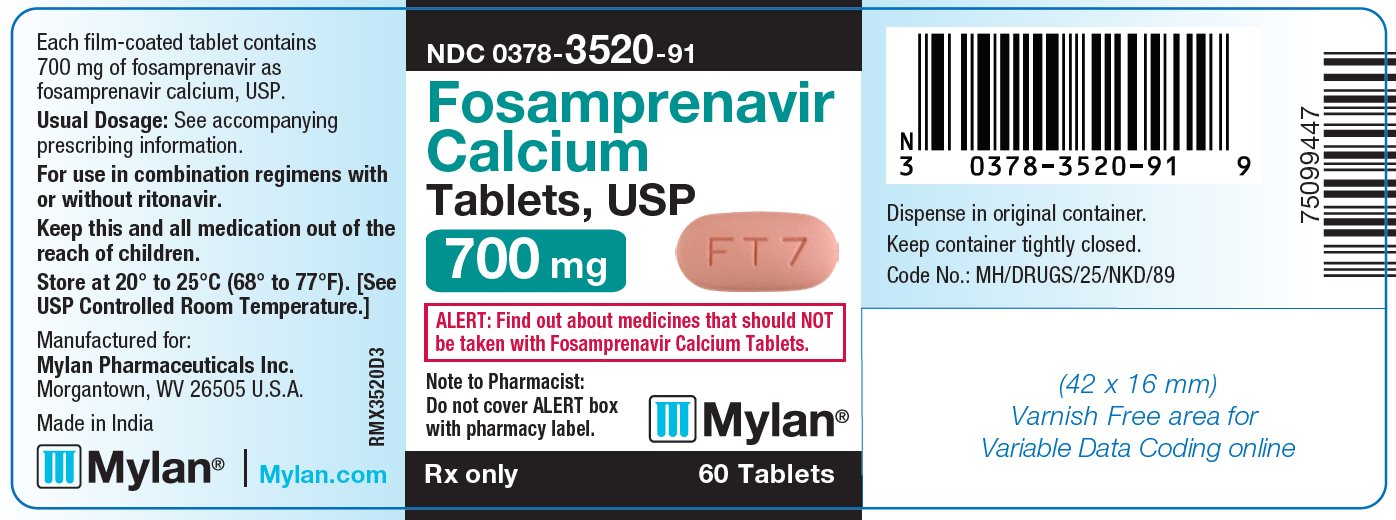
10PRINCIPAL DISPLAY PANEL – 700 mg
NDC 0378-3520-91
Fosamprenavir
Calcium
Tablets, USP
700 mg
Calcium
Tablets, USP
700 mg
ALERT: Find out about medicines that should NOT
be taken with Fosamprenavir Calcium Tablets.
be taken with Fosamprenavir Calcium Tablets.
Note to Pharmacist:
Do not cover ALERT box
with pharmacy label.
Do not cover ALERT box
with pharmacy label.
Rx only 60 Tablets
Each film-coated tablet contains
Usual Dosage: See accompanying
prescribing information.
prescribing information.
For use in combination regimens with
or without ritonavir.
or without ritonavir.
Keep this and all medication out of the
reach of children.
reach of children.
Store at 20° to 25°C (68° to 77°F). [See
USP Controlled Room Temperature.]
USP Controlled Room Temperature.]
Manufactured for:
Made in India
Mylan.com
RMX3520D3
Dispense in original container.
Keep container tightly closed.
Code No.: MH/DRUGS/25/NKD/89