Finasteride
What is Propecia (Finasteride)?
For many men, hair loss can be more than a cosmetic concern, it can affect self-esteem, confidence, and overall emotional well-being. Propecia (finasteride) offers an effective medical option for men experiencing male pattern hair loss, helping them regain not just hair, but also confidence in their appearance.
Propecia is a prescription medication used to treat androgenetic alopecia (male pattern baldness) in adult men. It belongs to a class of drugs known as 5-alpha-reductase inhibitors, which influence hormone levels related to hair growth. Unlike topical treatments, Propecia works from within the body to slow hair loss and, in many cases, promote regrowth. Approved by the U.S. Food and Drug Administration (FDA) in 1997, Propecia remains one of the most studied and widely prescribed oral medications for male hair loss today.
What does Propecia do?
Propecia is prescribed to treat male pattern hair loss, a common condition caused by a combination of genetics and hormonal changes. Over time, hair follicles in certain areas of the scalp shrink and produce thinner, shorter hairs eventually leading to visible baldness, especially on the crown and hairline.
Propecia works to slow down or stop further hair loss and can stimulate regrowth in some men. Clinical studies have shown that continuous use of finasteride leads to improved hair density and prevents further loss in up to 90% of men who take it for at least one year (NIH, 2024). The benefits are usually seen after several months of consistent treatment, and continued use is necessary to maintain results.
While Propecia does not cure baldness or restore hair in completely bald areas, it can significantly delay progression and help men preserve their existing hair longer, improving appearance and confidence.
How does Propecia work?
Propecia contains finasteride, which works by targeting the root hormonal cause of male pattern baldness. In men, testosterone is naturally converted into a more potent hormone called dihydrotestosterone (DHT) by an enzyme known as 5-alpha-reductase.
In genetically susceptible men, DHT causes hair follicles particularly those at the front and crown of the scalp to shrink over time. This process, known as miniaturization, results in thinner hair strands and shorter growth cycles until follicles stop producing hair altogether.
Propecia blocks the action of the 5-alpha-reductase enzyme, reducing DHT levels in the scalp by up to 60–70%. Lower DHT levels help protect hair follicles from shrinking, allowing them to continue producing healthy, thicker hair.
Clinically, this mechanism is significant because it addresses the underlying hormonal driver of male pattern hair loss, rather than just treating surface symptoms. This makes Propecia one of the few therapies proven to both prevent further hair loss and promote regrowth in men.
Propecia side effects
Most men tolerate Propecia well, but as with all medications, side effects can occur. Understanding them helps patients make informed decisions and feel reassured during treatment.
Common side effects may include:
- Decreased sex drive (libido)
- Difficulty achieving or maintaining an erection
- Reduced semen volume
These effects are typically mild and reversible after stopping the medication or with continued use as the body adjusts. Only a small percentage of men (around 2–4%) experience these symptoms (FDA, 2023).
Serious but rare side effects include:
- Persistent sexual side effects after discontinuation (post-finasteride syndrome — very uncommon and still under study)
- Breast tenderness, enlargement, or lumps
- Testicular pain
- Signs of allergic reaction such as rash, itching, or swelling
Men should seek medical attention if they experience breast changes, swelling of lips or face, or depression or anxiety while on the medication.
Who should avoid Propecia:
- Women and children should not take or handle crushed or broken tablets, as exposure can cause birth defects in unborn male babies.
- Men with known allergies to finasteride or similar drugs should not use it.
Doctors may periodically review a patient’s overall health, monitor hormone-related symptoms, and evaluate treatment response to ensure safe and effective long-term use.
Propecia dosage
Take Propecia once daily, by mouth, with or without food. Consistent daily use for 3-6 months shows visible improvement. Benefits reverse within 12 months if discontinued.
Doctors may advise follow-ups to track treatment and side effects. While no routine blood tests are needed, some clinicians may check PSA levels as finasteride can lower them.
Older adults can generally use Propecia safely, though it is not typically prescribed for men over 65 because male pattern baldness progression usually stabilizes naturally at that age.
Does Propecia have a generic version?
Yes. Finasteride is the generic version of Propecia, and it is FDA-approved. Generic finasteride tablets contain the same active ingredient, dosage strength, and therapeutic effect as brand-name Propecia. The main difference lies in appearance, inactive ingredients, and cost.
Generic finasteride offers an affordable, effective, and safe alternative to Propecia for hair loss. Finasteride is also sold as Proscar, a higher-strength version for enlarged prostate (BPH); patients should confirm they receive the correct formulation for hair loss.
Conclusion
Propecia (finasteride) is a trusted, scientifically proven treatment for male pattern baldness that helps men preserve their hair and confidence over time. By blocking the hormone responsible for follicle shrinkage, it slows hair loss and promotes visible regrowth in many users.
Propecia is generally safe and effective for male hair loss when prescribed and monitored by a doctor. Consistent use is crucial for gradual, long-term results. Always consult your doctor before starting or stopping treatment and maintain open communication.
References
- U.S. Food and Drug Administration (FDA). (2023). Propecia (finasteride) prescribing information. Retrieved from https://www.accessdata.fda.gov
- Mayo Clinic. (2024). Finasteride (oral route) drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Finasteride: Uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
- National Institutes of Health (NIH). (2024). Finasteride and androgenetic alopecia clinical studies. Retrieved from https://www.nih.gov
Approved To Treat
Top Global Experts
There are no experts for this drug
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
- Pregnancy. Finasteride use is contraindicated in women when they are or may potentially be pregnant. Because of the ability of Type II 5α-reductase inhibitors to inhibit the conversion of testosterone to 5α-dihydrotestosterone (DHT), finasteride may cause abnormalities of the external genitalia of a male fetus of a pregnant woman who receives finasteride. If this drug is used during pregnancy, or if pregnancy occurs while taking this drug, the pregnant woman should be apprised of the potential hazard to the male fetus.
- Hypersensitivity to any component of this medication.
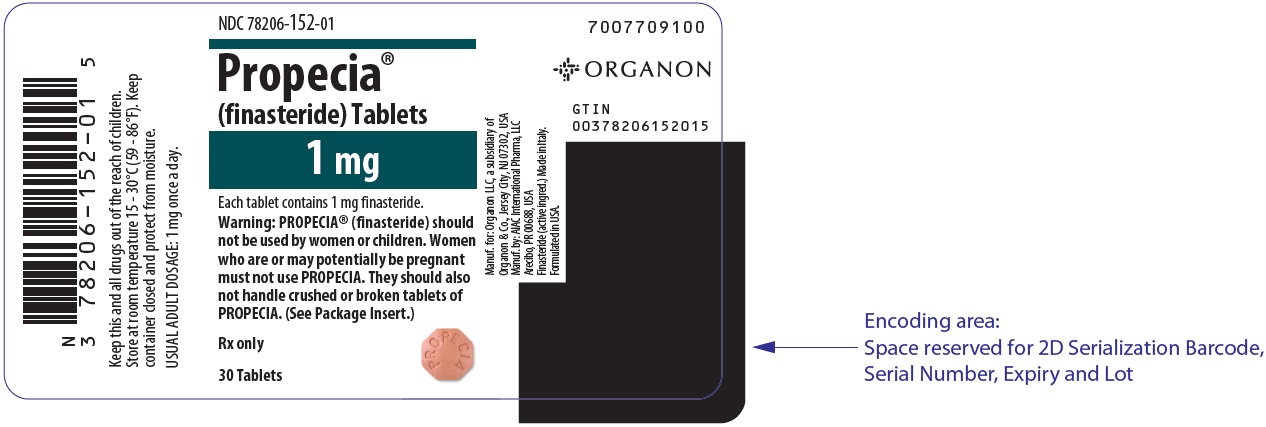
- NDC 78206-152-01 bottles of 30 (with desiccant)
- NDC 78206-152-02 PROPAK® bottles of 90 (with desiccant).