Nerlynx
What is Nerlynx (Neratinib)?
Finishing initial breast cancer treatment can feel both hopeful and uncertain. Many patients worry about the cancer returning and want every possible safeguard to protect their future health. Nerlynx (neratinib) is designed to reduce this risk. It offers a powerful follow-up option for certain women with breast cancer, helping extend the benefits of prior therapy and giving added peace of mind during recovery.
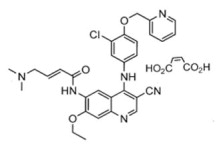
Nerlynx is an oral targeted therapy used in the treatment of HER2-positive breast cancer, a type of cancer driven by the overactivity of the HER2 protein. It belongs to a class of medications known as tyrosine kinase inhibitors (TKIs), which work by blocking specific enzymes that promote cancer cell growth. Approved by the U.S. Food and Drug Administration (FDA) in 2017, Nerlynx is often prescribed as an extended adjuvant therapy, meaning it is taken after initial treatments like surgery, chemotherapy, and trastuzumab (Herceptin) to lower the chance of the cancer coming back.
What does Nerlynx do?
Nerlynx is used to help prevent the recurrence of early-stage HER2-positive breast cancer in adults who have already completed a course of trastuzumab-based therapy. It is also used in some cases of metastatic breast cancer when the disease has spread beyond the breast often in combination with other medications such as capecitabine.
HER2-positive breast cancers tend to grow and spread more aggressively than other types because the HER2 protein stimulates rapid cell growth. By targeting this specific pathway, Nerlynx helps to stop or slow down cancer cell activity, reducing the risk of relapse after successful initial treatment.
Clinical studies have shown meaningful results. In the ExteNET trial, patients who took Nerlynx after completing trastuzumab had a significantly lower rate of cancer recurrence compared to those who received a placebo (NIH, 2024). This improvement was particularly notable among women with hormone receptor–positive (HR+) breast cancer, who often benefit the most from extended therapy.
For many patients, Nerlynx represents an important next step, one that reinforces the progress made through earlier treatments and supports long-term remission.
How does Nerlynx work?
Nerlynx works by blocking HER2 activity and related receptors on cancer cells. These receptors are proteins on the cell surface that send signals telling the cells to grow and divide. In HER2-positive breast cancer, these signals are overactive, leading to uncontrolled tumor growth.
Nerlynx contains neratinib, a tyrosine kinase inhibitor that binds to and permanently blocks the HER2 receptor, and two other similar proteins called HER1 (EGFR) and HER4. By inhibiting these receptors, Nerlynx disrupts the communication pathways that cancer cells rely on to multiply.
In simpler terms, the medication helps “turn off” the cancer’s growth signals, preventing it from spreading and reducing the chance of it returning.
Clinically, this mechanism is important because it provides continuous suppression of HER2 signaling even after other HER2-directed treatments, like trastuzumab, are completed. This “extended blockade” is what makes Nerlynx effective as a follow-up therapy in maintaining remission and preventing relapse.
Nerlynx side effects
Like many cancer treatments, Nerlynx can cause side effects, though not everyone experiences them, and many can be managed effectively with support from a healthcare team.
Common side effects include:
- Diarrhea (often occurring early in treatment)
- Nausea or vomiting
- Fatigue
- Abdominal pain
- Decreased appetite
- Rash or dry skin
- Mouth sores
Because diarrhea is the most frequent side effect, doctors often recommend preventive medications (antidiarrheals) when starting Nerlynx to reduce severity. Patients are encouraged to drink plenty of fluids and report prolonged or severe diarrhea immediately, as it can lead to dehydration if left untreated.
Less common but serious side effects may include:
- Liver problems (signs include yellowing of the skin or eyes, dark urine, or abdominal pain)
- Severe dehydration
- Allergic reactions (rash, swelling, difficulty breathing)
Before Nerlynx, tell your doctor about liver disease, GI issues, or medication allergies. Liver function tests are standard during treatment. Most patients tolerate Nerlynx well after initial side effects subside, often within the first month, enabling successful completion of therapy with monitoring.
Nerlynx dosage
Nerlynx is an oral tablet taken once daily with food, typically for one year to treat early-stage breast cancer. Consistent daily intake is crucial; missed doses should not be doubled, and patients should consult their healthcare provider.
Doctors will monitor liver function and overall health during therapy to detect side effects. Patients should log symptoms to help the care team adjust supportive measures. Older adults may need closer monitoring, but dose adjustments depend on treatment tolerance, not just age.
Does Nerlynx have a generic version?
As of 2025, Nerlynx (neratinib) does not have a generic version available in the United States. It is currently marketed only under the brand name Nerlynx by Puma Biotechnology, Inc. However, international versions may exist in other markets.
Generic versions, with the same active ingredient, dosage, and safety profile, are being developed by international manufacturers as patents expire. Patients can inquire about manufacturer-offered patient assistance programs to reduce costs.
Conclusion
Nerlynx (neratinib) offers a vital layer of protection for people recovering from HER2-positive breast cancer, helping to reduce the risk of recurrence and extend the benefits of earlier treatments. As an oral targeted therapy, it gives patients a sense of control and continuity during their post-treatment journey.
Manageable side effects like diarrhea or fatigue can be handled with proactive care and medical support, requiring regular follow-ups, lab monitoring, and open communication with healthcare providers for safe and successful treatment. Nerlynx aims to reduce cancer recurrence worries, offering an empowering and effective long-term breast cancer management step under oncology team guidance.
References
- U.S. Food and Drug Administration (FDA). (2024). Nerlynx (neratinib) prescribing information. Retrieved from https://www.accessdata.fda.gov
- National Institutes of Health (NIH). (2024). HER2-positive breast cancer and targeted therapy overview. Retrieved from https://www.nih.gov
- Mayo Clinic. (2024). Neratinib (oral route) patient drug information. Retrieved from https://www.mayoclinic.org
- MedlinePlus. (2024). Neratinib: Uses, side effects, and precautions. National Library of Medicine. Retrieved from https://medlineplus.gov
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This ComboMATCH patient screening trial is the gateway to a coordinated set of clinical trials to study cancer treatment directed by genetic testing. Patients with solid tumors that have spread to nearby tissue or lymph nodes (locally advanced) or have spread to other places in the body (advanced) and have progressed on at least one line of standard systemic therapy or have no standard treatment t...
Summary: This phase II ComboMATCH treatment trial compares the effect of neratinib to the combination of neratinib and palbociclib in treating patients with HER2 positive solid tumors. Neratinib and palbociclib are in a class of medications called kinase inhibitors. They work by blocking the action of an abnormal protein that signals cancer cells to multiply. This helps slow or stop the spread of tumor cel...
Summary: The purpose of this research is to look at the safety and effectiveness of a HER2-targeted therapy neratinib when given with capecitabine, a chemotherapy, for breast cancer patients with brain metastases whose tumors were HER2-negative by standard tests but showed abnormal HER2 activity based on the CELsignia results.
Related Latest Advances
Brand Information
- Diarrhea
- Hepatotoxicity
- Bottles of 180 tablets: NDC 70437-240-18
- Bottles of 133 tablets: NDC 70437-240-33
- Bottles of 126 tablets: NDC 70437-240-26
- Inform patients that NERLYNX has been associated with diarrhea, which may be severe in some cases.
- When not using dose escalation, instruct patients to initiate antidiarrheal prophylaxis with the first dose of NERLYNX.
- When using dose escalation, instruct patients to initiate 2 weeks of lower dose NERLYNX prior to receiving the recommended full dose of NERLYNX.
- Instruct patients to maintain 1–2 bowel movements per day and on how to use antidiarrheal treatment regimens.
- Advise patients to inform their healthcare provider immediately if severe (≥Grade 3) diarrhea or diarrhea associated with weakness, dizziness, or fever occurs during treatment with NERLYNX
- Inform patients that NERLYNX has been associated with hepatotoxicity which may be severe in some cases.
- Inform patients that they should report signs and symptoms of liver dysfunction to their healthcare provider immediately
- Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy
- Advise females of reproductive potential to use effective contraception during treatment and for 1 month after receiving the last dose of NERLYNX
- Advise lactating women not to breastfeed during treatment with NERLYNX and for at least 1 month after the last dose
- NERLYNX may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products
- NERLYNX may interact with gastric acid reducing agents. Advise patients to avoid concomitant use of proton pump inhibitors. When patients require gastric acid reducing agents, use an H
- NERLYNX may interact with grapefruit. Advise patients to avoid taking NERLYNX with grapefruit products
- For patients undergoing extended adjuvant treatment for early-stage breast cancer, instruct patients to take NERLYNX with food at approximately the same time each day consecutively until disease recurrence or for up to one year.
- For patients undergoing treatment for metastatic breast cancer, instruct patients to take NERLYNX with food on days 1–21 of a 21-day cycle, with capecitabine on Days 1–14 of a 21-day cycle until disease progression or unacceptable toxicities.
- If a patient misses a dose, instruct the patient not to replace the missed dose, and to resume NERLYNX with the next scheduled daily dose


