Beclomethasone Dipropionate
What is Qvar Redihaler (Beclomethasone Dipropionate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: TRICOLON is an investigator initiated, prospective, interventional, open-label, randomized, real-world, multi-centre, 3-arms study in the Netherlands. The primary objective is to investigate in COPD patients if single-inhaler triple therapy (SITT) is superior to multi-inhaler triple therapy (MITT) in terms of adherence to inhaled corticosteroids (ICS) therapy and to investigate if SITT with e-heal...
Summary: The goal of this clinical trial is to learn if metformin and antifibrotic drugs (pirfenidone) can modulate fibrosis and improve treatment outcomes in patients with oral submucous fibrosis (OSF). The study also aims to investigate the molecular mechanisms underlying their effects on exosome secretion and protein expression. The main questions it aims to answer are: Do metformin and antifibrotic dru...
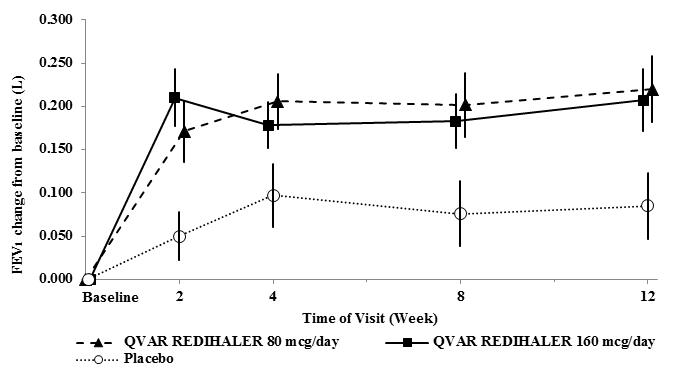
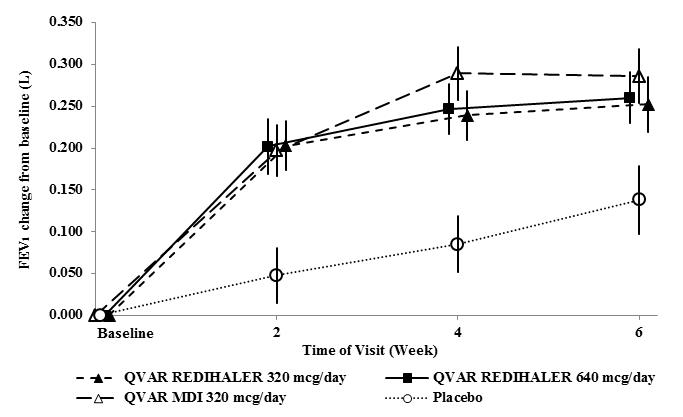
Summary: The mainstay of asthma treatment are ICSs, mostly combined with LABA. In the past decade LAMA had been accepted as an add-on treatment for patients on GINA treatment Steps 4 and 5. Recently, RCTs proved the efficacy and safety of fixed triple combinations of moderate and high dose of ICS and LABA, LAMA in a very selected asthmatic population, resulting in the market authorisation of these products...
Related Latest Advances
Brand Information
- 40 mcg in an aluminum canister contained within a beige plastic actuator and a hinged white cap
- 80 mcg in an aluminum canister contained within a maroon plastic actuator and a hinged white cap
- the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required
- in patients with known hypersensitivity to beclomethasone dipropionate or any of the ingredients in QVAR REDIHALER
- Oropharyngeal candidiasis
- Immunosuppression and risk of infections
- Hypercorticism and adrenal suppression
- Reduction in bone mineral density
- Growth effects
- Glaucoma and cataracts
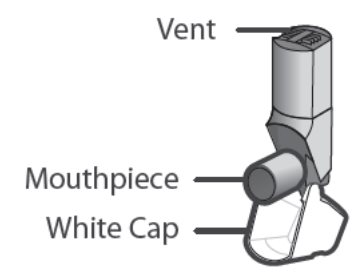
- There is no button. You must close the white cap to prepare the inhaler with medicine
- Do not shake. This breath-actuated device does not need to be shaken. This is not a press-and-breathe inhaler.
- Do not prime QVAR REDIHALER. The inhaler does not need to be primed.
- Do not use a spacer or volume holding chamber with QVAR REDIHALER.
- Always use the inhaler in the upright position (with the mouthpiece down).
- After the inhaler is prepared, it will deliver 1 inhalation of medicine when you breathe in (inhale) through the mouthpiece. Your dose might require more than 1 inhalation.
- Do not open the white cap or leave it open unless you are ready for your next inhalation. If the cap has been opened for more than 2 minutes or left in the open position, you will need to close the white cap before use.
- Do not breathe out or blow into any part of the inhaler. Breathing out or blowing into the inhaler can damage it.
- Do not suddenly stop using your QVAR REDIHALER. Contact your healthcare provider immediately if you stop using your QVAR REDIHALER.
- the inhaler body with the mouthpiece.
- the white cap that covers the mouthpiece of the inhaler.
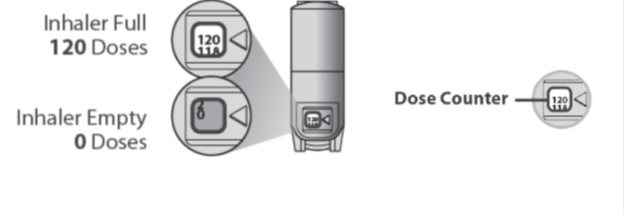
- Your QVAR REDIHALER contains 120 inhalations.
- The counter on the back of your inhaler shows how many inhalations you have left. When there are 20 inhalations left, the numbers in the dose counter will change to red and you should refill your prescription or ask your healthcare provider for another prescription.
- When the dose counter shows ‘0’, the background will turn solid red and your inhaler is empty. You should stop using the inhaler and throw it away.
- The white cap must be closed to prepare the inhaler before each inhalation or you will not receive your medicine. See Figure C.
- If the white cap is open, close the white cap to prepare your inhaler and look at the dose counter window to make sure that your inhaler is not empty.
- Do not open the cap until you are ready to take your inhalation.
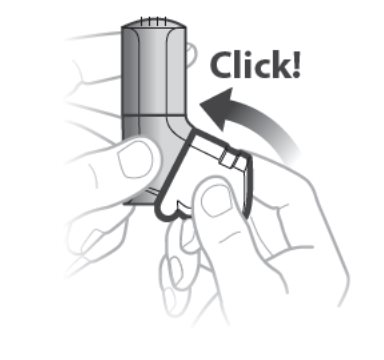
- Do not open the cap until you are ready to take your inhalation.
- Never breathe out or blow into the inhaler. Breathing out or blowing into the inhaler can damage it.
- Hold the inhaler upright as you take your inhalation.
- Store QVAR REDIHALER at room temperature between 68ºF to 77ºF (20ºC to 25ºC). Avoid exposure to extreme heat or cold.
- Keep the white cap on the inhaler closed during storage.
- Keep your QVAR REDIHALER inhaler dry and clean at all times.
- If you drop your QVAR REDIHALER, inspect it for damage before use. If the QVAR REDIHALER is damaged,
- Do not use or store your QVAR REDIHALER near heat or open flame. Exposure to temperatures above 120ºF (49ºC) may cause the canister to burst.
- Do not throw QVAR REDIHALER into fire or an incinerator.
- Throw away QVAR REDIHALER when the dose counter displays ‘0,’ or after the expiration date on the package, whichever comes first.
- Keep your QVAR REDIHALER and all medicines out of the reach of children.
- Do not wash or put any part of your QVAR REDIHALER in water.
- Clean the mouthpiece of your QVAR REDIHALER weekly with a clean, dry tissue or cloth.
- If you have any questions about QVAR REDIHALER or how to use your inhaler, go to www.QVAR.com or call 1-888-483-8279.





