Generic Name
Bimatoprost
Brand Names
Durysta, Lumigan, Latisse
FDA approval date: January 26, 2009
Classification: Prostaglandin Analog
Form: Implant, Solution
What is Durysta (Bimatoprost)?
LUMIGAN ®
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
Brand Information
DURYSTA (bimatoprost)
1INDICATIONS AND USAGE
DURYSTA
2DOSAGE FORMS AND STRENGTHS
Intracameral implant containing 10 mcg of bimatoprost in a drug delivery system.
3ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Implant migration
- Hypersensitivity
- Corneal adverse reactions
- Macular edema
- Intraocular inflammation
- Pigmentation
- Endophthalmitis
3.1Clinical TrialsExperience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common ocular adverse reaction observed in two randomized, active-controlled clinical trials with DURYSTA in patients with OAG or OHT was conjunctival hyperemia, which was reported in 27% of patients. Other common ocular adverse reactions reported in 5-10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, and iritis. Ocular adverse reactions occurring in 1-5% of patients were anterior chamber cell, lacrimation increased, corneal edema, aqueous humor leakage, iris adhesions, ocular discomfort, corneal touch, iris hyperpigmentation, anterior chamber flare, anterior chamber inflammation, and macular edema. The following additional adverse drug reactions occurred in less than 1% of patients: hyphema, iridocyclitis, uveitis, corneal opacity, corneal thickening, product administered at inappropriate site, corneal decompensation, cystoid macular edema, and drug hypersensitivity.
The most common nonocular adverse reaction was headache, which was observed in 5% of patients.
3.2PostmarketingExperience
The following adverse reactions have been identified during postapproval use of DURYSTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye disorders: endophthalmitis
4DESCRIPTION
DURYSTA is a sterile intracameral implant containing 10 mcg of bimatoprost, a prostaglandin analog, in a solid polymer sustained-release drug delivery system (DDS). The drug delivery system consists of poly (D,L-lactide), poly (D,L-lactide-co-glycolide), poly (D,L-lactide) acid end, and polyethylene glycol 3350. DURYSTA is preloaded into a single-use, DDS applicator to facilitate injection of the rod-shaped implant directly into the anterior chamber of the eye. The chemical name for bimatoprost is (
![The chemical name for bimatoprost is (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 415.57. Its molecular formula is C25H37NO4.](https://dailymed.nlm.nih.gov/dailymed/image.cfm?name=durysta-03.jpg&setid=3f59da84-0bcc-4c84-b3e2-e215681ef341)
Bimatoprost is a white to off-white powder, soluble in ethyl alcohol and methyl alcohol and slightly soluble in water. The polymer matrix slowly degrades to lactic acid and glycolic acid.
5CLINICAL STUDIES
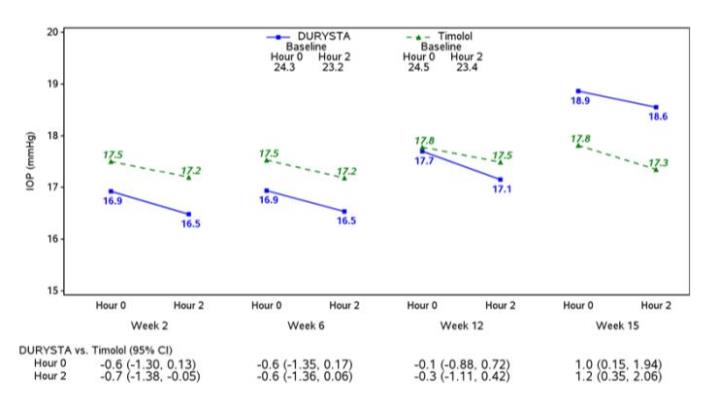
Efficacy was evaluated in two multicenter, randomized, parallel-group, controlled 20-month (including 8-month extended follow-up) studies of DURYSTA compared to twice daily topical timolol 0.5% drops, in patients with OAG or OHT. DURYSTA demonstrated an IOP reduction of approximately 5-8 mmHg in patients with a mean baseline IOP of 24.5 mmHg (see Figures 3 and 4).
Figure 3: Study 1 Mean IOP (mmHg) by Treatment Group and Treatment Difference in Mean IOP
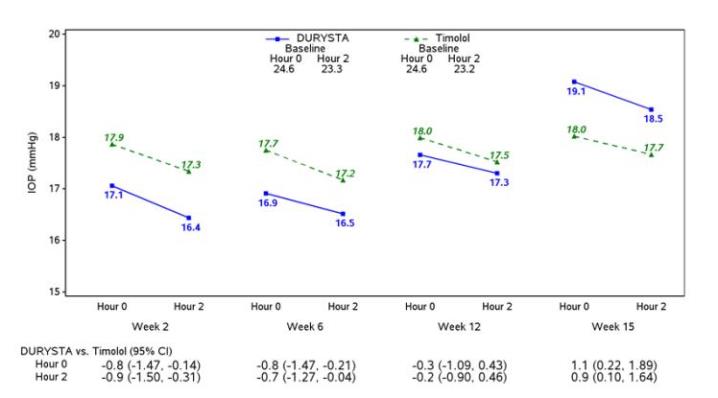
Figure 4: Study 2 Mean IOP (mmHg) by Treatment Group and Treatment Difference in Mean IOP
6HOW SUPPLIED/STORAGE AND HANDLING
DURYSTA contains a 10 mcg bimatoprost intracameral implant in a single-use applicator that is packaged in a sealed foil pouch containing desiccant, NDC 0023-9652-01.
Storage
Store refrigerated at 2°C to 8°C (36°F to 46°F).
7PATIENT COUNSELING INFORMATION
Treatment-related Effects
Advise patients about the potential risk for complications including, but not limited to, the development of corneal adverse events, intraocular inflammation or endophthalmitis
Potential for Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent
When to Seek Physician Advice
Advise patients that if the eye becomes red, sensitive to light, painful, or develops a change in vision, they should seek immediate care from an ophthalmologist
Distributed by: AbbVie Inc.
© 2024 AbbVie. All rights reserved.
V2.0USPI9652
8PRINCIPAL DISPLAY PANEL
NDC 0023-9652-01
