Brand Name
XYWAV
Generic Name
oxybates
View Brand Information FDA approval date: November 02, 2020
Classification: Central Nervous System Depressant
Form: Solution
What is XYWAV (oxybates)?
XYWAV is a central nervous system depressant indicated for the treatment of: Cataplexy or excessive daytime sleepiness in patients 7 years of age and older with narcolepsy.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
XYWAV (calcium, magnesium, potassium, and sodium oxybates)
WARNING: CENTRAL NERVOUS SYSTEM DEPRESSION and ABUSE AND MISUSE.
- Central Nervous System Depression
XYWAV is a CNS depressant. Clinically significant respiratory depression and obtundation may occur in patients treated with XYWAV at recommended doses - Abuse and Misuse
The active moiety of XYWAV is oxybate or gamma-hydroxybutyrate (GHB). Abuse or misuse of illicit GHB, either alone or in combination with other CNS depressants, is associated with CNS adverse reactions, including seizure, respiratory depression, decreases in the level of consciousness, coma, and death Because of the risks of CNS depression and abuse and misuse, XYWAV is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the XYWAV and XYREM REMS
1DOSAGE FORMS AND STRENGTHS
XYWAV is a clear to slightly opalescent oral solution at a total salt concentration of 0.5 g per mL. Each mL contains 0.5 g of total salts present as 0.234 g calcium oxybate, 0.096 g magnesium oxybate, 0.13 g potassium oxybate, and 0.04 g sodium oxybate (equivalent to 0.413 g total oxybate).
2CONTRAINDICATIONS
XYWAV is contraindicated for use in:
- combination with sedative hypnotics
- combination with alcohol
- patients with succinic semialdehyde dehydrogenase deficiency
3ADVERSE REACTIONS
The following clinically significant adverse reactions appear in other sections of the labeling:
- CNS depression
- Abuse and Misuse
- Respiratory Depression and Sleep-Disordered Breathing
- Depression and Suicidality
- Other Behavioral or Psychiatric Adverse Reactions
- Parasomnias
3.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adult Patients with Narcolepsy
The safety of XYWAV was evaluated in a 16‑week double-blind placebo-controlled randomized-withdrawal study in patients with narcolepsy with cataplexy (Study 1), which was followed by an open-label extension phase lasting 24 weeks
Adverse Reactions Leading to Treatment Discontinuation in Study 1
In Study 1, 9 of 201 patients (4%) reported adverse reactions that led to withdrawal from the study (anxiety, decreased appetite, depressed mood, depression, fatigue, headache, irritability, nausea, pain in extremity, parasomnia, somnolence, and vomiting). The most common adverse reaction leading to discontinuation was nausea (1.5%). The majority of adverse reactions leading to discontinuation began during the first few weeks of treatment.
Commonly Observed Adverse Reactions
The most common adverse reactions in Study 1 (incidence ≥5% of XYWAV-treated patients) were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis, anxiety, and vomiting.
Adverse Reactions Occurring at an Incidence of 2% or Greater:
Table 4 lists adverse reactions observed in the open-label titration and stable dose periods of Study 1 that occurred at a frequency of 2% or greater in adult patients treated with XYWAV.
Pediatric Patients (7 Years of Age and Older) with Narcolepsy
In the pediatric clinical trial with Xyrem (same active moiety as XYWAV), 104 patients aged 7 to 17 years (37 patients aged 7 to 11 years; 67 patients aged 12 to 17 years) with narcolepsy received Xyrem for up to one year
Adverse Reactions Leading to Treatment Discontinuation
In the pediatric clinical trial with Xyrem, 7 of 104 patients reported adverse reactions that led to withdrawal from the study (hallucination, tactile; suicidal ideation; weight decreased; sleep apnea syndrome; affect lability; anger, anxiety, depression; and headache).
Adverse Reactions in the Xyrem Pediatric Clinical Trial
The most common adverse reactions (≥5%) were nausea (20%), enuresis (19%), vomiting (18%), headache (17%), weight decreased (13%), decreased appetite (9%), dizziness (8%), and sleepwalking (6%).
Additional information regarding safety in pediatric patients appears in the following sections:
- Respiratory Depression and Sleep-Disordered Breathing
- Depression and Suicidality
- Other Behavioral or Psychiatric Adverse Reactions
- Parasomnias
The overall adverse reaction profile of Xyrem in the pediatric clinical trial was similar to that seen in the adult clinical trial program. The safety profile in pediatric patients with XYWAV is expected to be similar to that of adult patients treated with XYWAV and to that of pediatric patients treated with Xyrem.
Adult Patients with Idiopathic Hypersomnia
The safety of XYWAV was evaluated in a double-blind placebo-controlled randomized‑withdrawal study in patients with IH (Study 2). This study consisted of an open‑label titration period (OL OTTP) up to 14 weeks, a stable-dose period (SDP) for 2 weeks, a double‑blind, placebo‑controlled, randomized-withdrawal period (DB RWP) for 2 weeks, and an open-label extension period for 24 weeks (all study periods up to 42 weeks)
Adverse Reactions Leading to Treatment Discontinuation in Study 2
In Study 2, across all study periods (excluding placebo during the DB RWP) (up to 42 weeks), 17 of 154 patients (11%) reported adverse reactions that led to withdrawal from the study (anxiety, nausea, insomnia, vomiting, fatigue, feeling abnormal, fall, decreased appetite, dizziness, paresthesia, tremor, parasomnia, confusional state, hallucination visual, and irritability). The most common adverse reaction leading to discontinuation was anxiety (3.2%). The majority of adverse reactions leading to discontinuation began during the first few weeks of treatment.
Commonly Observed Adverse Reactions
The most common adverse reactions in Study 2 (incidence ≥5% of XYWAV‑treated patients) in addition to those observed in Study 1 as most common were insomnia, dry mouth, fatigue, somnolence, and tremor.
The safety profile observed in Study 2 was similar to that of Study 1. Adverse reactions occurring in ≥2% of patients treated with XYWAV in the open-label titration and stable dose periods in Study 2 are shown in Table 5:
Additional Adverse Reactions
Adverse reactions observed in clinical studies with Xyrem (≥2%), but not observed in Study 1 or Study 2 at a frequency of higher than 2%, and which may be relevant for XYWAV:
Pain, pain in extremity, cataplexy, disturbance in attention, sleep paralysis, and disorientation.
3.2Postmarketing Experience
The following adverse reactions have been identified during postapproval use of sodium oxybate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Arthralgia, fall*, fluid retention, hangover, hypersensitivity, hypertension, memory impairment, nocturia, and vision blurred.
- * The sudden onset of sleep in patients taking sodium oxybate, including in a standing position or while rising from bed, has led to falls complicated by injuries, in some cases requiring hospitalization.
4DESCRIPTION
XYWAV oral solution contains oxybate, a CNS depressant. The chemical name of oxybate is gamma-hydroxybutyrate (GHB). XYWAV contains a mixture of calcium oxybate, magnesium oxybate, potassium oxybate, and sodium oxybate equivalent to 0.5 g/mL, which corresponds to 0.413 g/mL oxybate.
Each mL of XYWAV contains: 0.234 g calcium oxybate, Ca(C
The chemical structure is:
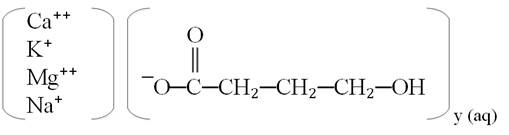
y=1 for Na+ and K+; y=2 for Mg2+ and Ca2+
The inactive ingredients are purified water and sucralose.
5PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Central Nervous System Depression
Inform patients and/or caregivers that XYWAV can cause central nervous system depression, including respiratory depression, hypotension, profound sedation, syncope, and death. Instruct patients not to engage in activities requiring mental alertness or motor coordination, including operating hazardous machinery, for at least 6 hours after taking XYWAV. Instruct patients and/or their caregivers to inform their healthcare providers of all the medications they take
Abuse and Misuse
Inform patients and/or caregivers that the active ingredient of XYWAV is gamma-hydroxybutyrate (GHB), which is associated with serious adverse reactions with illicit use and abuse
XYWAV and XYREM REMS
XYWAV is available only through a restricted program called the XYWAV and XYREM REMS
- XYWAV is dispensed only by the central pharmacy
- XYWAV will be dispensed and shipped only to patients enrolled in the XYWAV and XYREM REMS
XYWAV is available only from the central pharmacy participating in the program. Therefore, provide patients and/or caregivers with the telephone number and website for information on how to obtain the product.
Alcohol or Sedative Hypnotics
Advise patients and/or caregivers that alcohol and other sedative hypnotics should not be taken with XYWAV
Sedation
Inform patients and/or caregivers that the patient is likely to fall asleep quickly after taking XYWAV (often within 5 and usually within 15 minutes), but the time it takes to fall asleep can vary from night to night. The sudden onset of sleep, including in a standing position or while rising from bed, has led to falls complicated by injuries, in some cases requiring hospitalization
Administration Instructions
Inform patients to administer XYWAV at least 2 hours after eating. Inform patients and/or caregivers of patients taking XYWAV twice nightly, that the total nightly dosage of XYWAV is divided into two doses
Inform patients if their nightly dose requires multiple draws. Instruct patients on how to perform the draws from the bottle.
Respiratory Depression and Sleep-Disordered Breathing
Inform patients that XYWAV may impair respiratory drive, especially in patients with compromised respiratory function, and may cause apnea
Depression and Suicidality
Instruct patients and/or caregivers to contact a healthcare provider immediately if the patient develops depressed mood, markedly diminished interest or pleasure in usual activities, significant change in weight and/or appetite, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, or suicidal ideation
Other Behavioral or Psychiatric Adverse Reactions
Inform patients and/or caregivers that XYWAV can cause behavioral or psychiatric adverse reactions, including confusion, anxiety, and psychosis. Instruct them to notify their healthcare provider if any of these types of symptoms occur
Sleepwalking
Instruct patients and/or caregivers that XYWAV has been associated with sleepwalking and other behaviors during sleep, and to contact their healthcare provider if this occurs
Distributed By:
Jazz Pharmaceuticals, Inc.
Palo Alto, CA 94306
Protected by U.S. Patent Nos. 8,591,922; 8,772,306; 8,901,173; 9,050,302; 9,132,107; 9,486,426; 9,555,017; 10,195,168; 10,213,400; 10,675,258; 10,864,181; 11,253,494; 11,554,102; and 11,426,373.
6PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
