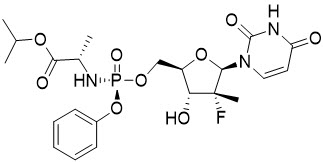
Sofosbuvir
What is Sovaldi (Sofosbuvir)?
Approved To Treat
Related Clinical Trials
Summary: The goal of this non-randomised, quasi-experimental, prospective comparative trial is to trial simplified care pathways for hepatitis C testing and treatment for people who inject drugs in Armenia, Georgia, and Tanzania. The main questions it aims to answer are: 1. What is the feasibility of implementing a hepatitis C simplified care and same-day treatment care model in community and harm reductio...
Summary: The goal of this clinical trial is to learn whether a low-barrier treatment program can help people with hepatitis C virus (HCV) who are in jail start and complete treatment more easily. This study focuses on adults at the Rhode Island Department of Corrections who have active HCV and are awaiting trial. The study asks: * Can a simplified, low-barrier HCV treatment program work in a jail setting? ...
Summary: This is a multicenter, single arm study of Sofosbuvir/Velpatasvir (SOF/VEL) for treatment of chronic hepatitis C infection during pregnancy. Treatment will be initiated during the second or third trimester in approximately 100 pregnant people. Maternal participants will take one SOF/VEL tablet once daily for 12 weeks (84 days) and followed until 12 weeks after treatment completion (postpartum). In...
Related Latest Advances
Brand Information
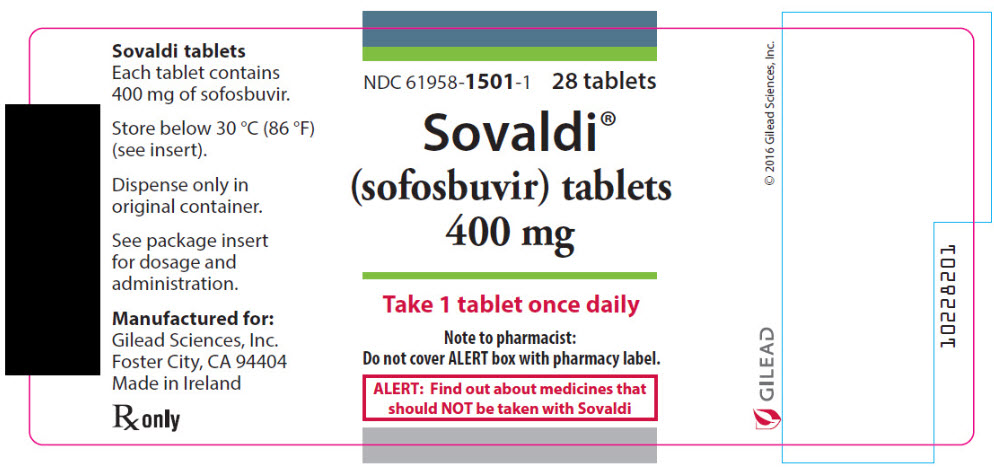
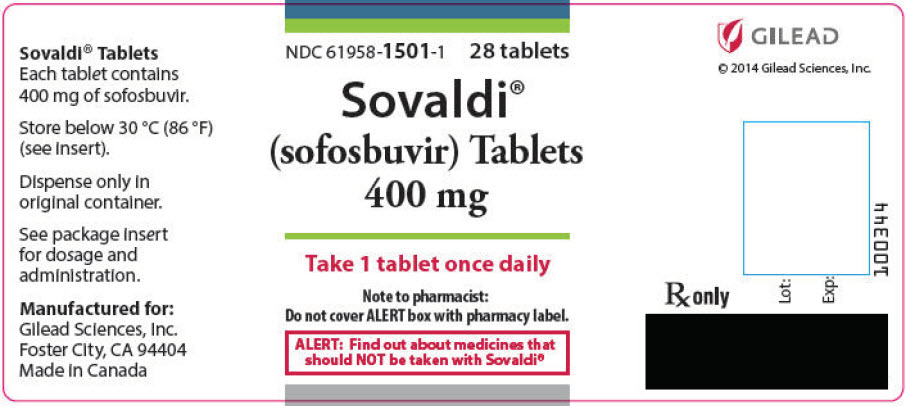
- 400 mg Tablets: 400 mg sofosbuvir: yellow, capsule-shaped, film-coated tablet debossed with "GSI" on one side and "7977" on the other side.
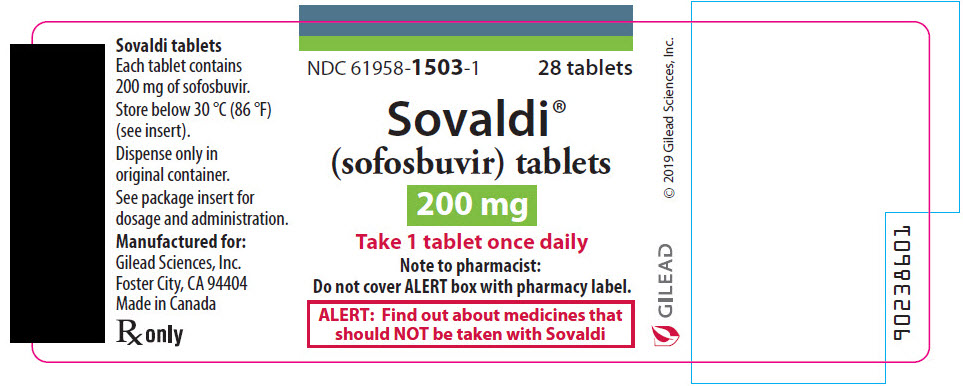
- 200 mg Tablets: 200 mg sofosbuvir: yellow, oval-shaped, film-coated tablet debossed with "GSI" on one side and "200" on the other side.
- 200 mg Pellets: 200 mg sofosbuvir: white to off-white pellets in unit-dose packets.
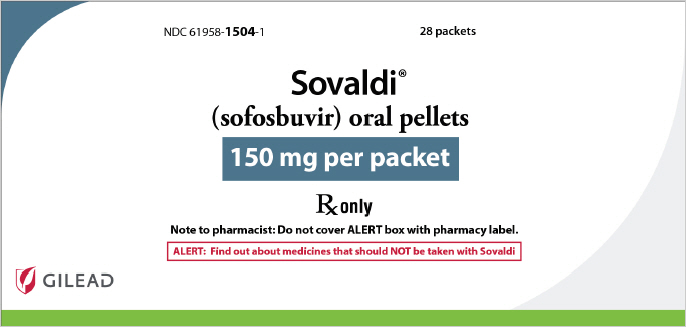
- 150 mg Pellets: 150 mg sofosbuvir: white to off-white pellets in unit-dose packets.
- Serious Symptomatic Bradycardia When Coadministered with Amiodarone
- For oral use only (take by mouth with or without food).
- Do not open the SOVALDI oral pellet packet(s) until ready to use.
- SOVALDI oral pellets are white to off-white pellets supplied as single-use packets in cartons. Each carton contains 28 packets.
- Do not use SOVALDI oral pellets if the carton tamper-evident seal, or the pellets packet seal, is broken or damaged.
- Daily SOVALDI oral pellet packet(s), as prescribed by your healthcare provider
- One or more spoonfuls of non-acidic soft food such as pudding, chocolate syrup, mashed potato, or ice cream
- Bowl
- Spoon
- Scissors (optional)
- Daily SOVALDI oral pellet packet(s), as prescribed by your healthcare provider
- Scissors (optional)
- Water (optional)
- Store SOVALDI pellets below 86°F (30°C).
- Throw away any unused portion. Do not store and reuse any leftover SOVALDI mixture (pellets mixed with food).